Dispensing system
A technology for viscous solutions and analgesics, which can be applied to dispensing devices, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as limiting the accuracy of distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
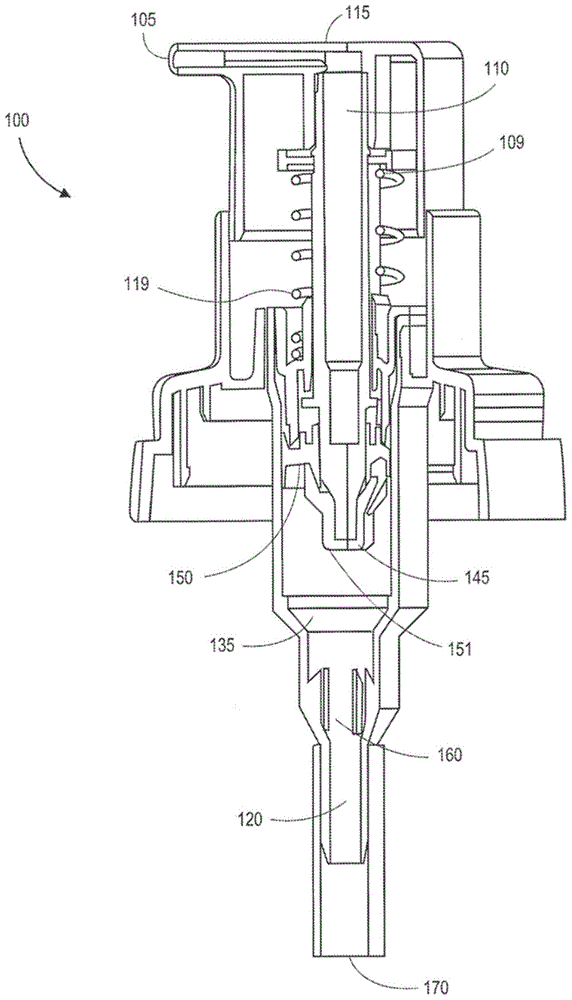
[0036] The present invention provides methods and systems for administering topical substances. In some aspects, methods and systems use unpressurized systems. In one embodiment, the present invention provides a method for administering a recommended dose of a topical analgesic in a viscous solution comprising:
[0037] Start the hand pump as described in:
[0038] a) compressing the spring with the rod attached thereto, and the rod slides relative to the piston, and into a metering chamber containing a first predetermined amount of topical analgesic in a viscous solution;
[0039] b) opening the outlet valve of the metering chamber, which allows a first predetermined amount of the topical analgesic in the viscous solution to flow into the channel or opening of the rod; and
[0040] c) When the limiter of the tappet is connected with the limiter of the piston, the piston is impacted, wherein a predetermined amount of topical analgesic in a viscous solution is discharged thro...
Embodiment 1
[0132] Example 1 shows the performance evaluation of a 100 mL Rexam Sof' Bag bottle with a 1 mL dispensing pump for topical diclofenac sodium 2.0% w / w.
[0133] 1. Summary
[0134] This current study was completed to evaluate the dose delivery properties of Rexam Sof'Bag containers with metered pumps for topical diclofenac sodium gel 2.0% w / w. 16 containers were studied over a period of 1 month (16 working days). The results show that when filled to full capacity, the container can consistently deliver the desired number of doses with high precision. The average unit dose weight delivered from each container varied from 0.97-0.99 g, which was well within 5% of the target dose (1.00 g). The unit dose weight standard deviation was stable at 0.01 g per vial.
[0135] 2. Introduction
[0136] Diclofenac Sodium Topical Gel 2.0% w / w is a drug that is being investigated for the treatment of signs and symptoms of knee osteoarthritis.
[0137] According to the FDA guidance documen...
Embodiment 2
[0185] Example 2: Clinical pharmacokinetic analysis of 2.0% w / w diclofenac sodium topical viscous solution (multiple doses)
[0186] A topical viscous solution of 2.0% w / w diclofenac sodium of the present invention was topically applied to both knees (2 mL [40.4 mg] / knee) every 12±0.5 hours for 7.5 consecutive days under fed conditions. Individuals dispense the solution from Hand Pump A of Example 1 and apply the topical viscous solution to clean dry skin. To avoid spillage, dispense 2 mL (2 pumps) of the topical viscous solution first into the hands and then onto the knees. Apply the topical viscous solution evenly around the front, back and sides of the knee. On the other knee, repeat, making sure to apply until completely dry.
[0187] The following pharmacokinetic parameters of diclofenac sodium were determined:
[0188] Day 1: Maximum observed plasma concentration (C max ), reaching C max time (T max ), and the area under the plasma concentration curve (AUC) for the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com