Method for preparing Eprosartan impurity EP12A
A kind of eprosartan, EP12A technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
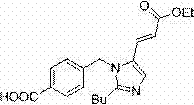
[0019] Synthesis of (E)-4-[[2-butyl-5-(2-ethoxycarbonylvinyl)imidazol-1-yl]methyl]benzoic acid [impurity EP12A]:
[0020] To a 500 mL three-neck flask, add 28.6g (0.10 mol) 4-[[(2-butyl-5-formyl) imidazol-1-yl] methyl] benzoic acid, 26.4g (0.20 mol) Monoethyl malonate, 150g toluene, 1.74g (0.02 mol) morpholine, started stirring, raised the temperature to reflux, separated water, and reacted for 12h. The reaction solution was concentrated under reduced pressure, and then purified by column chromatography to obtain 30.0 g of white solid, HPLC purity: 98%, yield: 84.3%. 1 H NMR (DMSO- d 6 ) δ 0.80 (t, J=4.0 Hz, 3H), 1.19 (t, J=4.0 Hz, 3H), 1.28 (m, 2H), 1.56 (m, 2H), 2.65 (t, J=4.0 Hz, 2H ), 4.10 (q, J = 4.0 Hz, 2H), 5.49 (s, 2H), 6.31 (d, J = 12.0 Hz, 1H), 7.08 (d, J = 7.0 Hz, 2H), 7.38 (d, J = 12.0 Hz, 1H), 7.70 (s, 1H), 7.94 (d, J = 7.0 Hz, 2H), 13.07 (b, 1H); 13 C NMR (DMSO- d 6 ) δ 14.1, 14.6, 22.1, 26.4, 29.7, 46.3, 60.3, 114.7, 126.3, 128.6, 130.3, 130.5, 130.9, 131...
Embodiment 2
[0022] Synthesis of (E)-4-[[2-butyl-5-(2-ethoxycarbonylvinyl)imidazol-1-yl]methyl]benzoic acid [impurity EP12A]:
[0023] To a 500 mL three-neck flask, add 28.6g (0.10 mol) 4-[[(2-butyl-5-formyl) imidazol-1-yl] methyl] benzoic acid, 26.4g (0.20 mol) Monoethyl malonate, a mixed solvent of 75g toluene and 75g xylene, 1.74g (0.02 mol) morpholine, started stirring, raised the temperature to reflux, separated water, and reacted for 12h. The reaction solution was concentrated under reduced pressure, and then purified by column chromatography to obtain 30.3 g of white solid, HPLC purity: 98%, yield: 85.0%. 1 H NMR (DMSO- d 6 ) δ 0.80 (t, J=4.0 Hz, 3H), 1.19 (t, J=4.0 Hz, 3H), 1.28 (m, 2H), 1.56 (m, 2H), 2.65 (t, J=4.0 Hz, 2H ), 4.10 (q, J = 4.0 Hz, 2H), 5.49 (s, 2H), 6.31 (d, J = 12.0 Hz, 1H), 7.08 (d, J = 7.0 Hz, 2H), 7.38 (d, J = 12.0 Hz, 1H), 7.70 (s, 1H), 7.94 (d, J = 7.0 Hz, 2H), 13.07 (b, 1H); 13 C NMR (DMSO- d 6 ) δ 14.1, 14.6, 22.1, 26.4, 29.7, 46.3, 60.3, 114.7, 126.3...
Embodiment 3
[0025] Synthesis of (E)-4-[[2-butyl-5-(2-ethoxycarbonylvinyl)imidazol-1-yl]methyl]benzoic acid [impurity EP12A]:
[0026] To a 500 mL three-neck flask, add 28.6g (0.10 mol) 4-[[(2-butyl-5-formyl) imidazol-1-yl] methyl] benzoic acid, 26.4g (0.20 mol) Monoethyl malonate, 180g toluene, 1.74g (0.02 mol) morpholine, started stirring, raised the temperature to reflux, separated water, and reacted for 12h. The reaction solution was concentrated under reduced pressure, and then purified by column chromatography to obtain 31.3 g of white solid, HPLC purity: 97%, yield: 88.0%. 1 H NMR (DMSO- d 6 ) δ 0.80 (t, J=4.0 Hz, 3H), 1.19 (t, J=4.0 Hz, 3H), 1.28 (m, 2H), 1.56 (m, 2H), 2.65 (t, J=4.0 Hz, 2H ), 4.10 (q, J = 4.0 Hz, 2H), 5.49 (s, 2H), 6.31 (d, J = 12.0 Hz, 1H), 7.08 (d, J = 7.0 Hz, 2H), 7.38 (d, J = 12.0 Hz, 1H), 7.70 (s, 1H), 7.94 (d, J = 7.0 Hz, 2H), 13.07 (b, 1H); 13 C NMR (DMSO- d 6 ) δ 14.1, 14.6, 22.1, 26.4, 29.7, 46.3, 60.3, 114.7, 126.3, 128.6, 130.3, 130.5, 130.9, 131...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com