Novel aminodithioformate compounds, and preparation method and application thereof
A technology of carbadithiocarbamic acid and ester compounds, which is applied in the field of new carbadithiocarbamate compounds and their preparation, and can solve the problems of limited and no anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
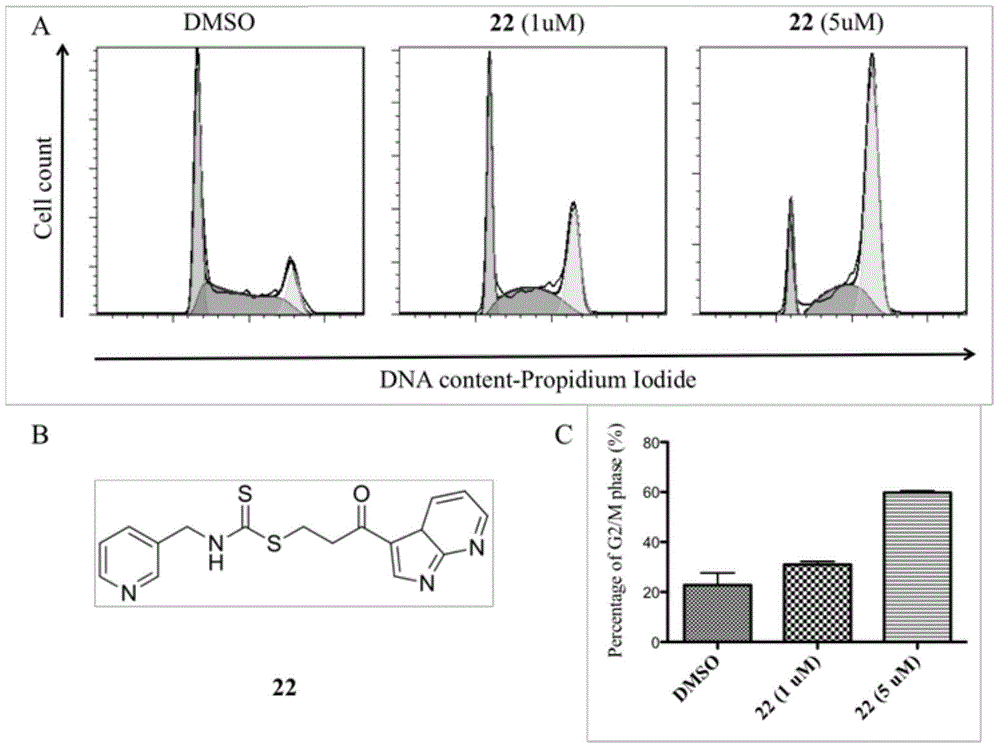
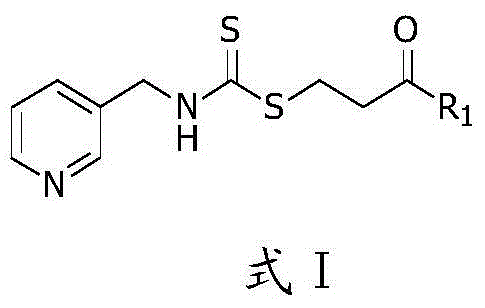
Image
Examples
Embodiment 1
[0047] Example 1 Synthesis of 3-pyridinemethylcarbamodithioate-(2-benzoyl)ethyl ester (compound 1)
[0048]
[0049] Dissolve 3-aminomethylpyridine (541mg, 5mmol) in 25mL DCM, add TEA (506mg, 5mmol), stir at room temperature for 10min, add CS 2 (457mg, 6mmol), reacted at room temperature for 20min, added 3-chloropropiophenone (843mg, 5mmol), reacted for 5h at room temperature, directly spin-dried the solvent under reduced pressure, column chromatography (P:E=1:2), and obtained white Solid, 77.2% yield. Melting point: 82-84°C.
[0050] 1 H NMR (400MHz, DMSO) δ10.50 (s, 1H), 8.70-8.32 (m, 2H), 7.97 (d, J = 7.4Hz, 2H), 7.66 (ddd, J = 10.8, 8.5, 4.4Hz, 2H), 7.53(t, J=7.1Hz, 2H), 7.37(dd, J=7.7, 4.8Hz, 1H), 4.86(d, J=5.3Hz, 2H), 3.50(dt, J=11.8, 4.7 Hz,4H).
[0051] 13 C NMR (100MHz, DMSO) δ198.72, 197.82, 149.56, 148.90, 136.65, 135.96, 133.90, 133.40, 129.25, 128.37, 123.98, 47.58, 38.65, 29.32.
Embodiment 2
[0052] Example 2 Synthesis of 3-pyridinemethylcarbamodithioate-[2-(3,4-dichloro-benzoyl)]ethyl ester (compound 2)
[0053]
[0054] Dissolve 3,4-dichloroacetophenone (756mg, 4mmol) in 10mL DMF, add paraformaldehyde (240mg, 8mmol), catalyst CF 3 COOH (iPr) 2 NH (860mg, 4mmol), react at 80°C for 12h, cool to room temperature, add 50mL of water, extract with ethyl acetate (10mL×3), combine the organic phases, dry over anhydrous sodium sulfate, concentrate, and perform column chromatography (P: E=20:1), the oily intermediate (2-1) was obtained with a yield of 27.5%. Dissolve 3-aminomethylpyridine (324mg, 3mmol) in 20mL DCM, add TEA (303mg, 3mmol), stir at room temperature for 5min, add CS 2 (274mg, 3.6mmol), reacted for 10min, added intermediate 2-1, reacted at room temperature for 5h, evaporated the solvent under reduced pressure, and column chromatography (P:E=2:1) gave a white solid with a yield of 56.3%. Melting point: 139.3-140.2°C.
[0055] Intermediate 2-1
[0056...
Embodiment 3
[0062] Example 3 Synthesis of 3-pyridinemethylcarbamodithioate-[2-(4-fluoro-benzoyl)]ethyl ester (compound 3)
[0063]
[0064] Using 4-fluoroacetophenone as a raw material, the target compound was synthesized with reference to the synthetic route of Example 2. Intermediate 3-1 is an oily product with a yield of 46.1%; product 3 is a white solid with a yield of 35.4% and a melting point of 127.4-128.1°C.
[0065] Intermediate 3-1
[0066]
[0067] 1 H NMR (400MHz, CDCl 3 )δ8.11-7.83(m,2H),7.14(dt,J=10.6,9.6Hz,3H),6.58-6.23(m,1H),6.12-5.77(m,1H).
[0068] 13 C NMR (100MHz, CDCl 3 )δ188.31, 165.96, 163.43, 132.63, 132.60, 130.98, 130.34, 130.25, 129.29, 114.85, 114.64.
[0069] Product 3
[0070] 1 H NMR (400MHz, DMSO) δ10.60(t, J=5.5Hz, 1H), 8.81-8.35(m, 2H), 8.33-7.92(m, 2H), 7.93-7.56(m, 1H), 7.46- 7.17(m,3H),4.85(d,J=5.6Hz,2H),3.72-3.40(m,4H).
[0071] 13 C NMR (100MHz, DMSO) δ197.73, 197.32, 166.84, 164.34, 149.57, 148.85, 135.98, 133.44, 131.45, 131.36, 12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com