Lesinurad-containing compound composition and preparation method thereof
A kind of technology of lysenoside and composition, applied in the field of preparing said composition, and can solve problems such as complicated process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
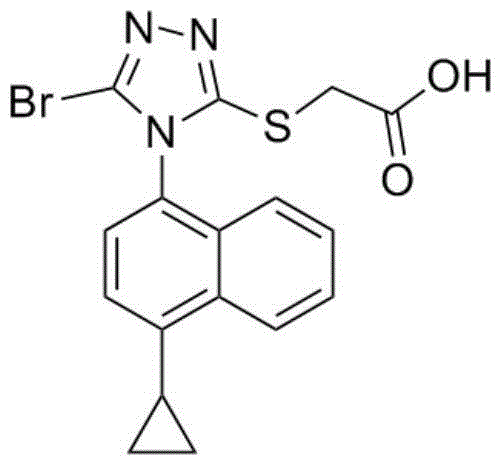
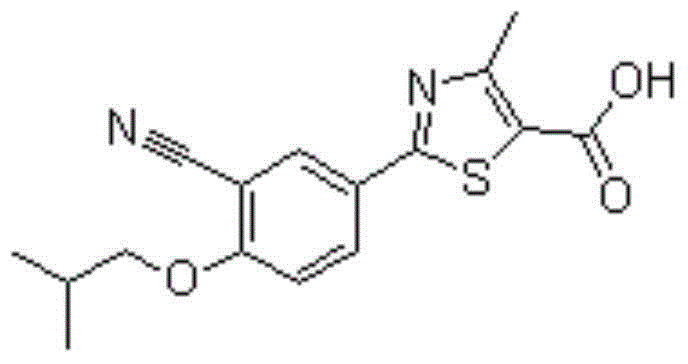
[0018] Example 1 Lesinorefebuxostat Compound Tablets
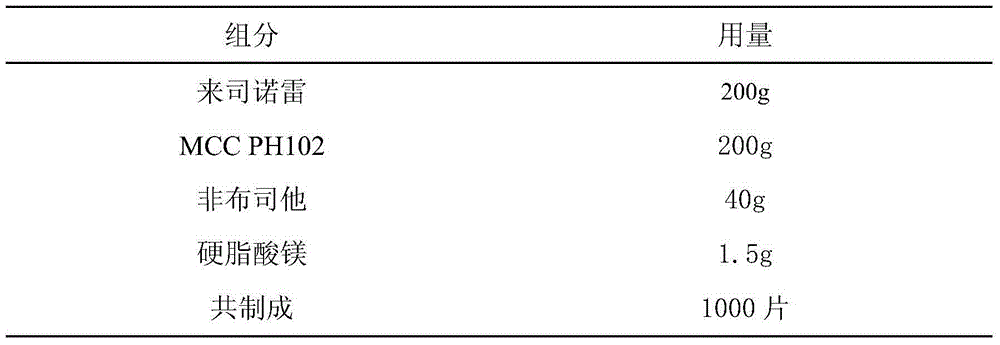
[0019] Lesinorefebuxostat Prescription:
[0020]
[0021] Preparation:
[0022] 1. Preparation of Lesnorefebuxostat Solid Composition
[0023] In the future, dissolve senorefebuxostat in ethanol respectively. After the active ingredient is completely dissolved, add MCC PH102 while stirring. After mixing evenly, volatilize the solution at 40 degrees Celsius for 10 minutes, and then rapidly raise the temperature to 65 degrees Celsius until Ethanol was evaporated completely, and then the solid composition was taken out. Pass through a 28-mesh sieve and granulate to obtain a solid composition.
[0024] 2. Preparation of Lesnorefebuxostat Tablets
[0025] Take the solid composition and add the lubricant magnesium stearate to compress the tablet to get Snorefebuxostat Tablets
Embodiment 2
[0026] Example 2 Lesnorefebuxostat Capsules
[0027] Prescription of Lesinorefebuxostat Capsules:
[0028]
[0029] Preparation:
[0030] 1. Preparation of Lesnorefebuxostat Solid Composition
[0031] In the future, dissolve senorefebuxostat in ethanol respectively. After the active ingredient is completely dissolved, add MCC PH102 while stirring. After mixing evenly, volatilize the solution at 40 degrees Celsius for 10 minutes, and then rapidly raise the temperature to 65 degrees Celsius until Ethanol was evaporated completely, and then the solid composition was taken out. Pass through a 28-mesh sieve and granulate to obtain a solid composition.
[0032] 2. Preparation of Lesnorefebuxostat Capsules
[0033] The solid composition is mixed with magnesium stearate to fill capsules.
Embodiment 3
[0034] Embodiment 3: lesinorefebuxostat granules
[0035] Prescription of Lesinorefebuxostat Capsules:
[0036]
[0037] 1. Preparation of Lesnorefebuxostat Solid Composition
[0038] In the future, dissolve senorefebuxostat in ethanol respectively. After the active ingredient is completely dissolved, add MCC PH102 while stirring. After mixing evenly, volatilize the solution at 40 degrees Celsius for 10 minutes, and then rapidly raise the temperature to 65 degrees Celsius until Ethanol was evaporated completely, and then the solid composition was taken out. Pass through a 28-mesh sieve and granulate to obtain a solid composition.
[0039] 2. Preparation of Lesnorefebuxostat Granules
[0040] The solid composition is dispensed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com