External preparation for treating corticosteroid-dependent dermatitis
A technology for dependent dermatitis and topical preparations, applied in the direction of medical preparations containing active ingredients, skin diseases, plant raw materials, etc., can solve the problems of unsatisfactory treatment effects, low overall cure rate, long treatment cycle, etc., to promote Skin cell metabolism, no toxic side effects, definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] The external preparation for treating hormone-dependent dermatitis in this example is composed of the following proportions by weight: 10 parts of peony root extract; 10 parts of comfrey extract; 5 parts of licorice extract; 100 parts of witch hazel extract; Snow lotus flower extract 5 parts; Astragalus extract 5 parts; Borneol 1 part; Glycerin: 5 parts; Bisabolol 10 parts; Allantoin: 1 part; Vitamin B3: 10 parts; 10 parts; L-vitamin C: 5 parts; L-glutamic acid: 10 parts.
Embodiment 2
[0012] The external preparation for treating hormone-dependent dermatitis in this embodiment is composed of the following proportions by weight: 40 parts of peony root extract; 40 parts of comfrey extract; 20 parts of licorice extract; 40 parts of witch hazel extract; Snow lotus extract 10 parts; Astragalus extract 40 parts; Borneol 3 parts; Glycerin: 20 parts; Bisabolol 20 parts; Allantoin: 10 parts, Vitamin B3: 40 parts; 20 parts; L-vitamin C: 20 parts; L-glutamic acid: 40 parts;
Embodiment 3
[0014] The external preparation for treating hormone-dependent dermatitis in this example is composed of the following proportions by weight: 40 parts of peony root extract; 40 parts of comfrey extract; 10 parts of licorice extract; 20 parts of witch hazel extract; 10 parts of snow lotus extract; 40 parts of astragalus extract; 1 part of borneol; 10 parts of glycerin; 10 parts of bisabolol; 10 parts of allantoin; 40 parts of vitamin B3; 20 parts; L-vitamin C: 5 parts; L-glutamic acid: 40 parts.
[0015] Embodiment effect:
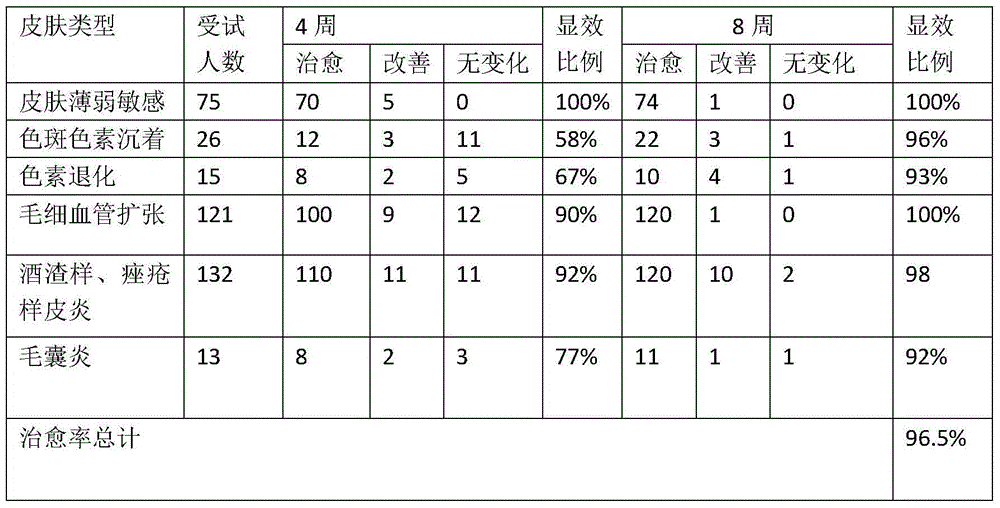
[0016] Through 8 weeks of regular observation and follow-up of 162 test subjects with hormone-dependent dermatitis, the use of 10-40 parts of peony root extract; 10-40 parts of comfrey extract; 5-20 parts of licorice extract; witch hazel extract 10-40 parts; 5-10 parts of snow lotus extract; 5-30 parts of astragalus extract; 1-3 parts of borneol; 5-20 parts of glycerin; 10-20 parts of bisabolol; vitamin B3: 10-40 parts; vitamin B2: 1-10 parts; vitamin E: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com