Huwentoxin-iv variants and methods of use
A tiger-striped tarantula and toxin technology, which can be applied to pharmaceutical formulations, medical preparations containing active ingredients, peptide/protein components, etc., and can solve problems such as no successful reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
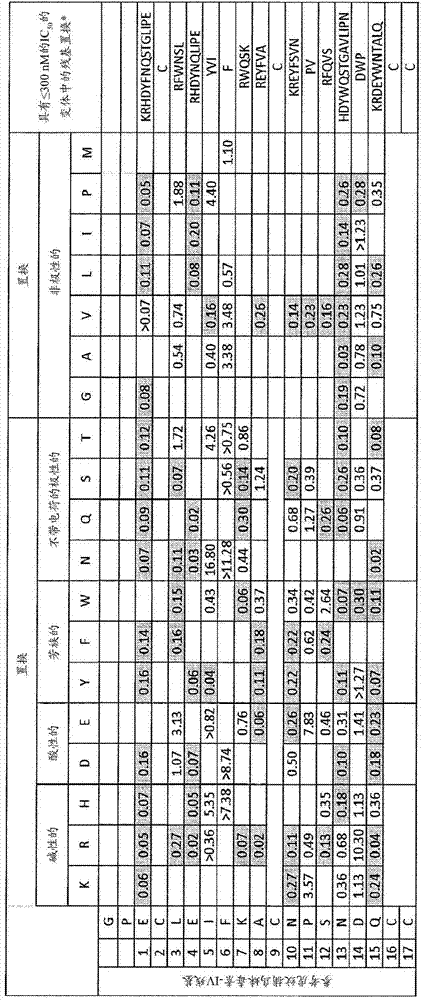
[0211] Design and Preparation of Huwen Tarantula Toxin-IV Variants
[0212] A single-site amino acid scanning library was prepared to replace the wild-type Ornithoctonus huwena derived from the venom of the Chinese tarantula Ornithoctonus huwena (ECLEIFKACNPSNDQCCKSSKLVCSRKTRWCKYQI; SEQ ID NO: 1). Ala, Asp, Glu, Phe, Gly, His, Ile, Lys, Leu, Asn, Pro, Gln, Arg, Ser, Thr, Val, Trp, and Tyr at each non-cysteine residue. Huwentoxin-IV variants are encoded as HRV3C protease-cleavable human serum albumin (HSA) fusion proteins from N-terminus to C-terminus in the form: His 6 -HSA-(GGGGS) 4 -HRV3C cleavage site -Huwentoxin-IV variant. Each variant peptide has a residual N-terminal GP from the cleavage site after cleavage from HSA and a C-terminal GK that is the endogenous amidation recognition sequence. Single-site variants were tested in a fluorescence-based screening assay measuring their ability to inhibit veratridine-induced membrane potential, and hits were confirmed in Q...
example 2
[0223] Example 2. Characterization of Huwentoxin-IV Variants
[0224] Membrane depolarization assay
[0225] use FRET assay (fluorescence resonance energy transfer) on Tetra using DISBAC2(3) (Invitrogen, K1018) as electron acceptor and PTS18 (8-octadecyloxypyrene-1,3,6-trisodium trisulfonate ) (Sigma) as a donor, by exciting the donor at 390-420nm and measuring FRET at 515-575nm, the prepared Huwentoxin-IV variants were measured to inhibit the Nav1.7 agonist veratridine (3-veratridine Ability of redrine; Biomol, Catalog #NA125) to induce membrane depolarization.
[0226] HEK293F cells stably expressing hNav1.7 channels under G418 selection (Invitrogen) were cultured in DMEM / F12 supplemented with glutamine, 10% FBS, 1% NEAA and 400 μg / ml G-418. 50 μl of harvested cells were plated at 25,000 cells / well in poly-lysine coated 384-well black clear bottom plates. Plates were incubated at room temperature (RT) for 15 min, followed by overnight incubation at 37°C. All incuba...
example 3
[0249] Example 3. Analgesic activity of Huwentoxin-IV following intraplantar administration in rats method
[0250] Male Sprague-Dawley (CD) rats (Charles River, San Diego) weighing >300 grams were used in this study. Naive animals were trained 2 days prior to the test day (in order to reduce variability in responses). Training consisted of performing the actual trials on each animal multiple times for each rat over a duration of -1 hour. Animals first received a sharpie mark proximal to the toes in the middle of the back of the left paw to enable consistent trials of the same position of the paw. The rat was then loosely wrapped in a towel, leaving the hind paw uncovered, and the left hind paw was placed in a Randall Selitto device (Ugo-Basile Randall-Selitto Device, Analgesy- Meter) with a Sharpie marker just below the cone of the test device that contacts the paw, and pressure is increased at a steady rate through an electronic ramp with foot control until the animal ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com