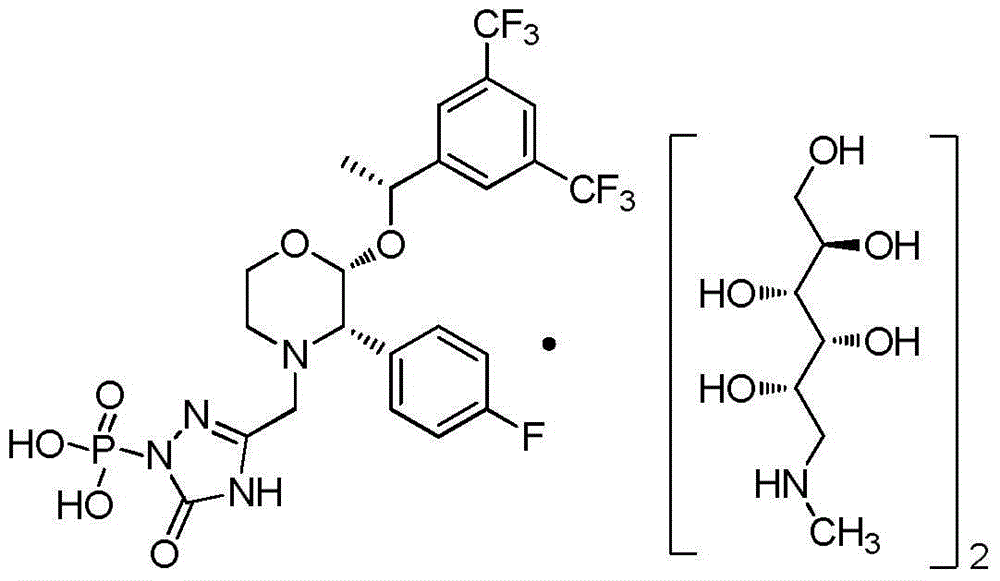
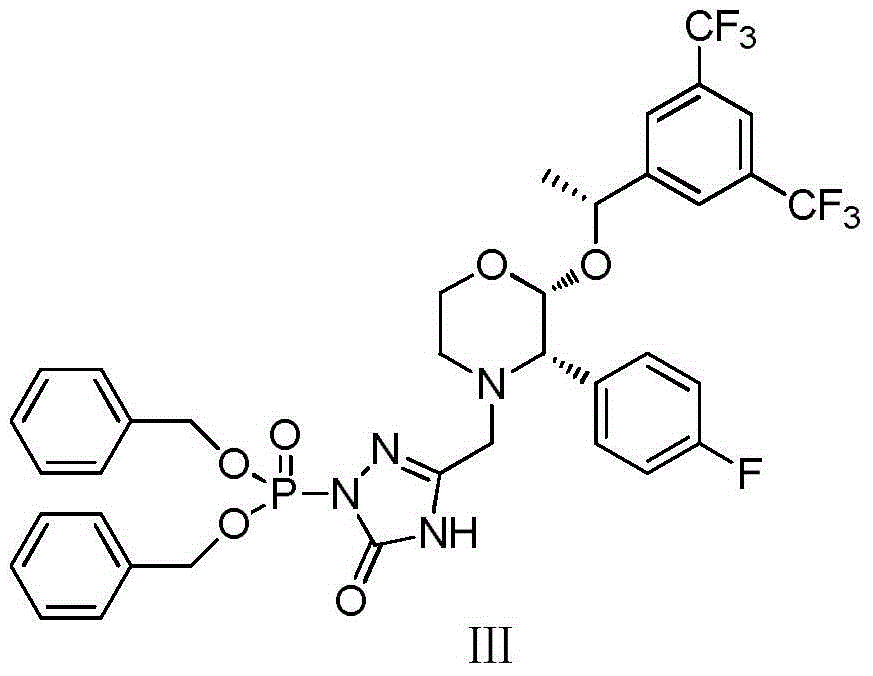
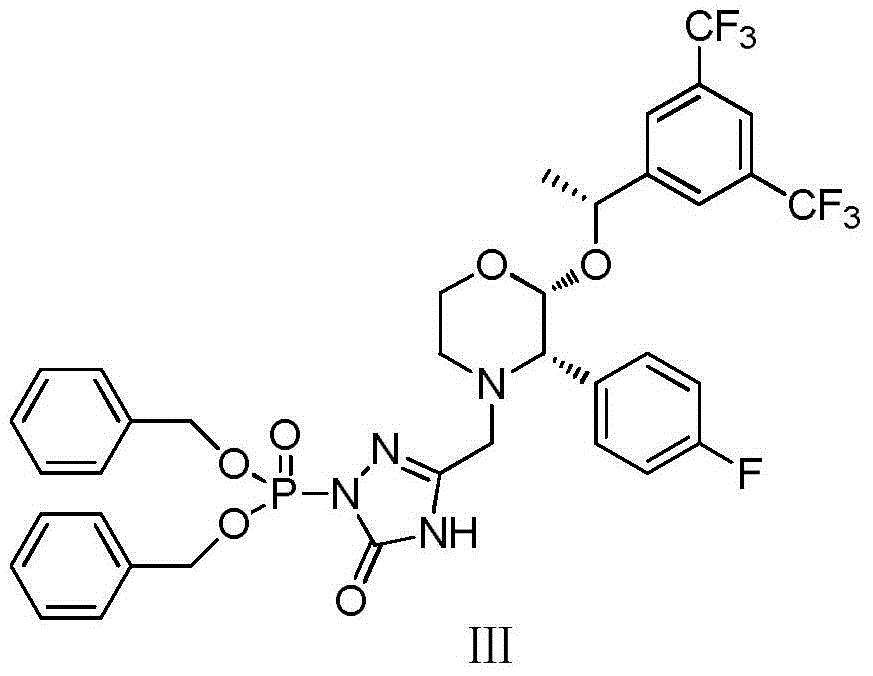
Method for preparing fosaprepitant dimeglumine intermediates
A technology of chloromethyl and step 2, which is applied in the fields of chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., which can solve the problems of unsuitable for industrial production, high price of aprepitant, and increased processing Difficulty and other issues, to achieve the effect of high implementation value, simple and efficient post-processing, and stable physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 13.35g (100mmol) 3-chloromethyl-1,2,4-triazolin-5-one and 50ml THF into the reaction flask, stir well, add 220ml of 1mol / L sodium hexamethyldisilazane The tetrahydrofuran solution was cooled to -5°C, and a tetrahydrofuran solution of dibenzylphosphoryl chloride (32.6g of dibenzylphosphoryl chloride dissolved in 50ml of tetrahydrofuran) was slowly added dropwise. After the dripping was complete, the stirring reaction was continued, and the reaction endpoint was monitored by TLC. Add 300ml of saturated aqueous sodium bicarbonate solution and 300ml of isopropyl ether to the reaction solution, stir for 15min, separate the liquids, wash the organic phase with saturated brine until neutral, dry over anhydrous sodium sulfate, filter, concentrate the filtrate to dryness, and dry in vacuo to obtain a white solid 35.84g, yield 91%.
[0033] The resulting 35.84g product, 42.64g (90mmol) [2R-2α(R), 3α]-2-[1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-( Add 4-fluorophenyl)-3-morp...
Embodiment 2
[0038] Add 13.35g (100mmol) 3-chloromethyl-1,2,4-triazolin-5-one, 64.62g (120mmol) tetrabenzyl pyrophosphate and 50mlTHF into the reaction flask, stir well, and cool down to - At 5°C, 220 ml of 1 mol / L sodium hexamethyldisilazane tetrahydrofuran solution was added dropwise. After the drop was completed, the stirring reaction was continued, and the reaction end point was monitored by TLC. Add 300ml of saturated aqueous sodium bicarbonate solution and 300ml of isopropyl ether to the reaction solution, stir for 15min, separate the liquids, wash the organic phase with saturated brine until neutral, dry over anhydrous sodium sulfate, filter, concentrate the filtrate to dryness, and dry in vacuo to obtain a white solid 34.25g, yield 87%.
[0039]The resulting 34.25g product, 40.75g (86mmol) [2R-2α(R), 3α]-2-[1-[3,5-bis(trifluoromethyl)phenyl]ethoxyl]-3-( Add 4-fluorophenyl)-3-morpholine hydrochloride and 100ml methanol into the reaction flask, stir to dissolve. Control the reactio...
Embodiment 3
[0041] Add 13.35g (100mmol) 3-chloromethyl-1,2,4-triazolin-5-one, 64.62g (120mmol) tetrabenzyl pyrophosphate and 50mlTHF into the reaction flask, stir well, and cool down to - At 5°C, 24.69 g (220 mmol) of potassium tert-butoxide was added. After the addition was complete, the stirring reaction was continued, and the end point of the reaction was monitored by TLC. Add 300ml of saturated aqueous sodium bicarbonate solution and 300ml of isopropyl ether to the reaction solution, stir for 15min, separate the liquids, wash the organic phase with saturated brine until neutral, dry over anhydrous sodium sulfate, filter, concentrate the filtrate to dryness, and dry in vacuo to obtain a white solid 33.86g, yield 86%.
[0042] The resulting 33.86g product, 40.27g (85mmol) [2R-2α(R), 3α]-2-[1-[3,5-bis(trifluoromethyl)phenyl]ethoxyl]-3-( Add 4-fluorophenyl)-3-morpholine hydrochloride and 100ml methanol into the reaction flask, stir to dissolve. Control the reaction temperature at 0°C, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com