Fluorine-containing syn-form novel nicotine compound and preparation method and application thereof
A compound and composition technology, applied in the field of new neonicotinoid insecticides, can solve the problems of affecting the insecticidal activity of compounds, narrow insecticidal spectrum, and low activity of lepidopteran pests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The preparation method of the compound of the present invention
[0013] The compounds of the present invention can be synthesized by the reaction steps described above. Those skilled in the art can synthesize intermediates in the reaction steps according to prior art documents;
[0014] The preparation method of the compound of the present invention can be the preparation method commonly used in this field, and the present invention provides a kind of preferred preparation method of the compound described in general formula (A), comprises method:
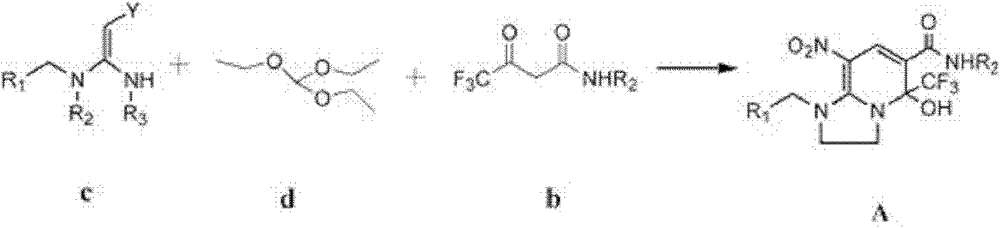
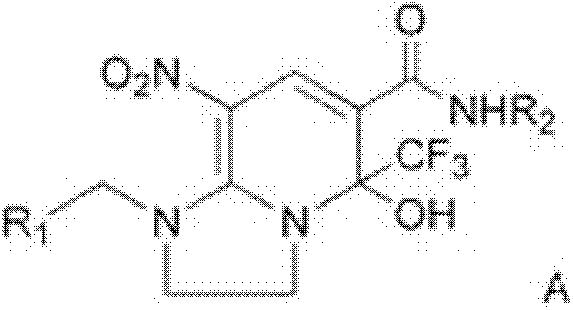
[0015] Under solvent-free conditions, the compound of formula (b), the compound of formula (c) and the compound of formula (d) are reacted to obtain the compound of formula (A):
[0016]
[0017] In the above formulas, R 1 , R 2 as defined in claim 1;
[0018] In a preferred example, the reaction temperature is 100-150°C, preferably 110-130°C.
[0019] Insecticidal activity of the active substance of the present ...
Embodiment 1
[0025] The compound synthesis in the embodiment is carried out according to the following steps:
[0026]
[0027] Add 5 mmol 2-chloro-5-(2-nitromethylene-imidazolidin-1-ylmethyl)-pyridine, 5 mL triethyl orthoformate, 6 mmol ethyl trifluoroacetoacetate aniline derivative into a 50 mL round bottom In the flask, reflux and stir for 3 hours. After cooling, filter with suction and wash with petroleum ether. The resulting solid product was separated by column chromatography (dichloromethane:methanol=10:1) to obtain a yellow powdery solid;
[0028] Compound experimental data:
[0029] Compound 1
[0030] Yield: 70%. Melting point: 184-185°C. 1 H NMR (CDCl 3 )δ(ppm): 8.58(s, 1H), 8.34(d, 1H, J=1.6Hz, Py-H), 8.11(s, 1H), 7.80(dd, 1H, J 1 =2.0Hz,J 2 =8.0Hz, Py-H), 7.54(s, 1H, Py-H), 7.53(s, 1H, Ph-H), 7.35(m, 3H, Ph-H), 7.17(t, 1H, J= 7.2Hz, Ph-H), 5.09(d, 1H, J=15.2Hz, Py-CH 2 ), 4.42 (d, 1H, J=15.6Hz, Py-CH 2 ), 4.21-3.63 (m, 4H, N-CH 2 -CH 2 -N).IR(KBr,cm -1 ): 3274...
Embodiment 2
[0061] Embodiment 2. The insecticidal activity test of compound of the present invention
[0062] Insecticidal activity against planthoppers
[0063] Planthoppers are homoptera pests with piercing and sucking mouthparts, and are a common crop pest. The brown planthopper (Nilaparvata lugens) was used as the test object, and the spray method was used to test;
[0064] Operation process: the compound to be tested is accurately prepared into a 500, 250 ppm solution with acetone as a solvent, and treated with acetone aqueous solution as a blank control. Each treatment was replicated 3 cups (ie replicated 3 times). Spray 2ml per cup evenly with a small manual sprayer. 6 hours before spraying, 10 rice planthoppers were inoculated in each pot. A total of 3 batches of tests were carried out. After 24 hours of treatment, the number of dead insects of the test insects was counted, and the mortality rate (%) was calculated. The results are shown in Table 1;
[0065] Mortality rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com