Clostridium perfringens enterotoxin positive bacteria dual fluorescent quantitative PCR rapid detection kit
A clostridium perfringens, detection kit technology, applied in the direction of determination/inspection of microorganisms, biochemical equipment and methods, etc., can solve the problem of non-involving Clostridium perfringens exotoxin cpa and enterotoxin cpe diagnostic technology and other problems, to achieve the effect of fast detection speed, high accuracy and low false positive rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Composition and preparation of double fluorescence quantitative PCR rapid detection kit for positive enterotoxin of Clostridium perfringens
[0032] 1. Reagent composition:
[0033] EasyTaq enzyme (5U / μL), dNTPs (10mM), were purchased from Promega Company; PCR primer pairs cpa01, cpa02 and cpe01, cpe02, and probes cpap, cpep were synthesized by Shanghai Sangong Bioengineering Company;
[0034] 2. Reagent preparation
[0035] A) Fluorescence quantitative reaction solution: 1×PCR Buffer (containing Mg 2+), dNTPs 0.5mM, Taq enzyme 2U, upstream primer cpa01 and downstream primer cpa02 of Clostridium perfringens exotoxin cpa 0.4μM each, fluorescent probe cpap 0.4μM, upstream of Clostridium perfringens enterotoxin cpe The primer cpe01 and the downstream primer cpe02 were 0.4 μM each, and the fluorescent probe cpep was 0.4 μM. Among them, the primer sequence of exotoxin cpa, upstream primer cpa01: 5'-GATTTGTAAGGCGCTTATTTGT-3', downstream primer cpa02: 5'-ATAGCATGAGTTCCTGTTC...
Embodiment 2
[0040] Clostridium perfringens enterotoxin-positive dual fluorescent quantitative PCR rapid detection primers and methods of use of the kit
[0041] 1. Sample processing
[0042] The sample to be tested is a tissue or liquid sample: According to the national standard method, aseptically take 10g (mL) of the sample and put it into 90mL of 0.5% buffered peptone water, shake and mix well, then take the sample liquid and inoculate it in TSC medium for bacterial enrichment, 37 ℃ anaerobic culture for 24h.
[0043] 2. The processing of the tested samples adopts the rapid boiling method to extract the genomic DNA of the bacteria, which is used as a template for the fluorescent quantitative PCR reaction. Pick a single colony on the TSC medium, put it into 200 μl of sterilized deionized water, heat and boil for 15 minutes, take out the test tube, centrifuge at 10,000 rpm for 5 minutes, and take the supernatant as a template for fluorescent quantitative PCR reaction.
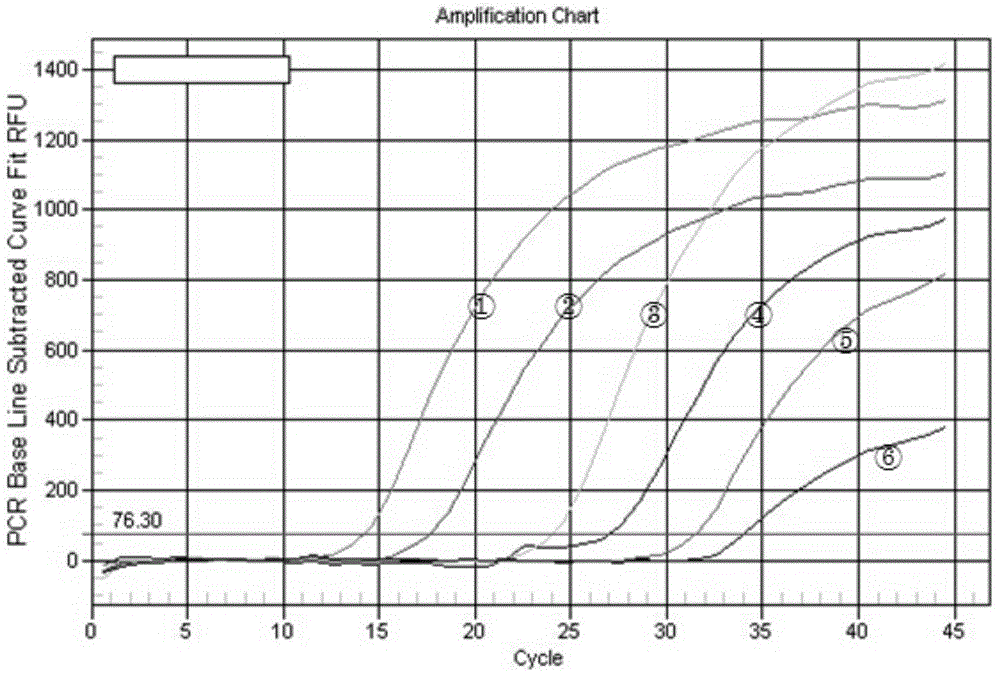
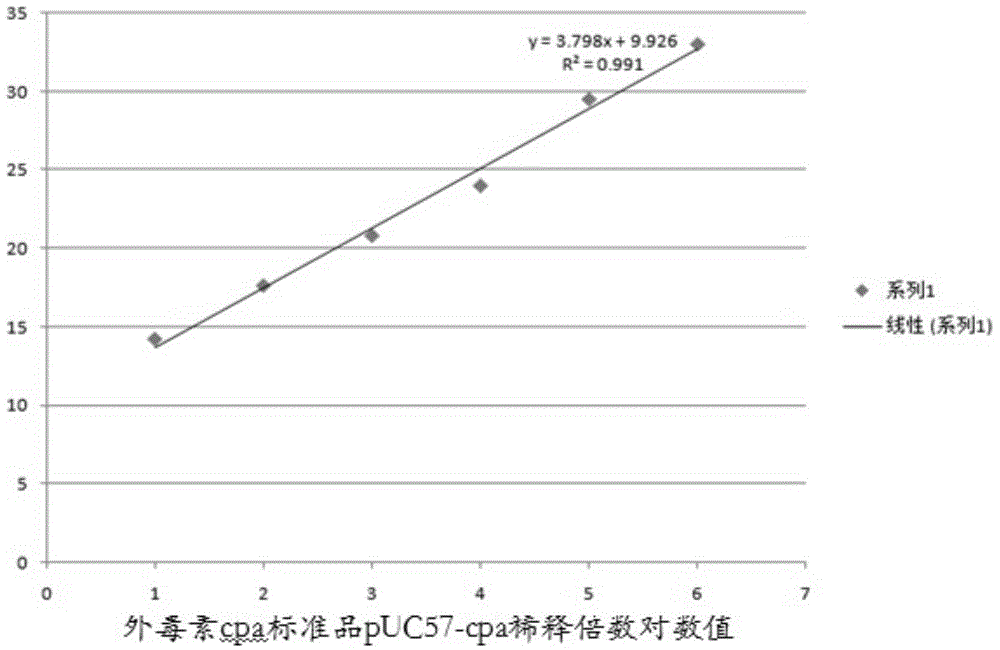
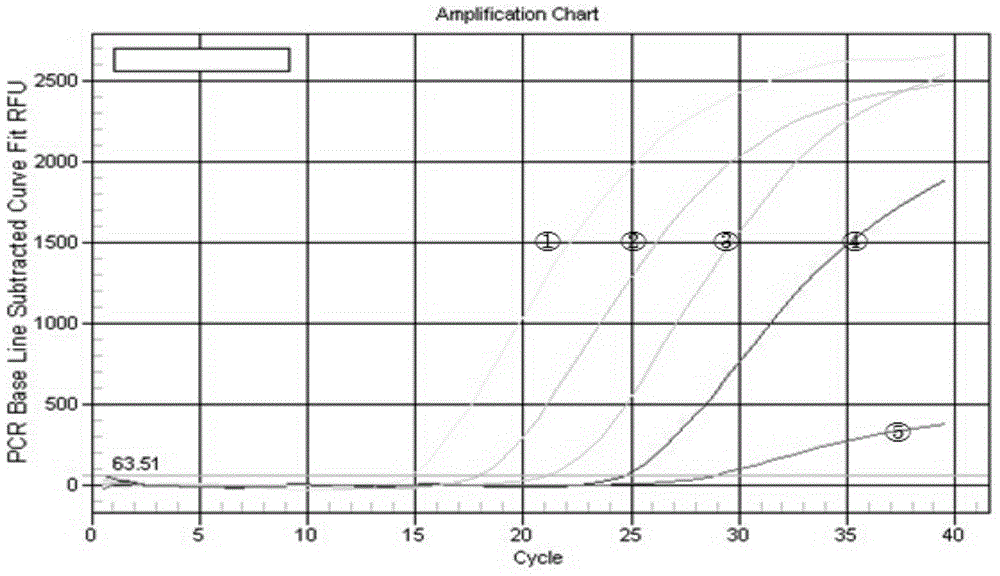
[0044] 3. Fluor...
Embodiment 3
[0056] Application of double fluorescent quantitative PCR rapid detection primers and kits for Clostridium perfringens enterotoxin positive
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com