Clostridium perfringens alpha toxin humanized single chain antibody 7d
A clostridium perfringens, single-chain antibody technology, applied in the direction of antibodies, antidote, anti-enzyme immunoglobulin, etc. The effect of low molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Construction of recombinant expression plasmid pET-28a-CPA
[0019] According to the complete sequence of Clostridium perfringens alpha toxin (CPA) published in Genbank (Genbank accession number L43548.1), the complete gene sequence of Clostridium perfringens alpha toxin was designed and synthesized. The NdeI and EcoRI restriction sites were inserted into the ends, and cloned into pMD19-T vector to construct plasmid pMD19-T-CPA, which was then transferred into E.coliJM109 to make puncture strains for storage.
[0020] The large fragment of the carrier plasmid pET-28a purified and recovered after digestion and the CPA digestion product purified and recovered after digestion were ligated with T4 DNA ligase to obtain the recombinant plasmid pET-28a-CPA, which was transformed into E. coli JM109 competent cells, containing kan-resistant agar plates were used for preliminary screening. A single colony was selected and cultured in LB liquid medium; the plasmid was ...
Embodiment 2
[0021]Example 2: Expression, purification and protein properties of CPA
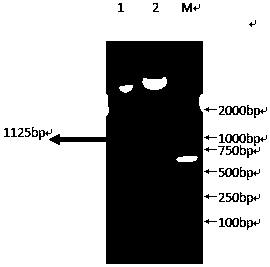
[0022] The recombinant plasmid pET-28a-CPA was transformed into the expression bacteria-Escherichia coli BL21 (DE3), and the expression was induced by IPTG. The results of SDS-PAGE showed that the recombinant protein CPA had an obvious expression band around 43KD, and the size was consistent with the theoretical value. The expression amount accounted for 15.6% of the total bacterial protein (see figure 2 ).
[0023] The supernatant and the precipitate of the recombinant protein expressing bacteria lysate were subjected to SDS-PAGE electrophoresis at the same time. The results showed that most of the target protein was in the supernatant, and a small amount of target protein also existed in the corresponding position in the precipitate. can be expressed in a soluble form under ambient conditions (see image 3 ).
[0024] Purification of the recombinant protein CPA, the recombinant protein expression b...
Embodiment 3
[0025] Example 3: Screening of fully human anti-C. perfringens alpha toxin neutralizing scFv antibodies
[0026] The purified CPA recombinant protein was coated on a 96-well microtiter plate as an antigen, overnight at 4°C. The supernatant was discarded the next day, blocked with 2% Milk-PBS at 37°C for 2 h, and the prepared phage antibody library Source bioscience (UK), purchased from Beijing Ximei Technology Co., Ltd. (China distributor), was added to the phage antibody library at a titer of 1.0 × 10 13 , incubate with vigorous shaking for 60 min at room temperature, and let stand for 60 min. Then, the liquid was discarded and washed 10 times with PBS containing 0.1% Tween-20. After washing, the remaining liquid in each well was gently patted dry, and 50 µL of eluate (5 mg / mL trypsin-PBS) was added to each well. Shake vigorously for 10 min at room temperature to elute the phage and collect and store at 4°C.
[0027] E. coli TG1 was infected with the eluted phage and spread...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com