A kind of synthetic technology of cefoxitin
A technology for the synthesis of cefoxitin acid, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of high cost, difficulty in obtaining cephalosporanic acid, and low yield, reduce energy consumption and discharge of organic waste water, and be suitable for large-scale industrialization Effects on production, improved yield and product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
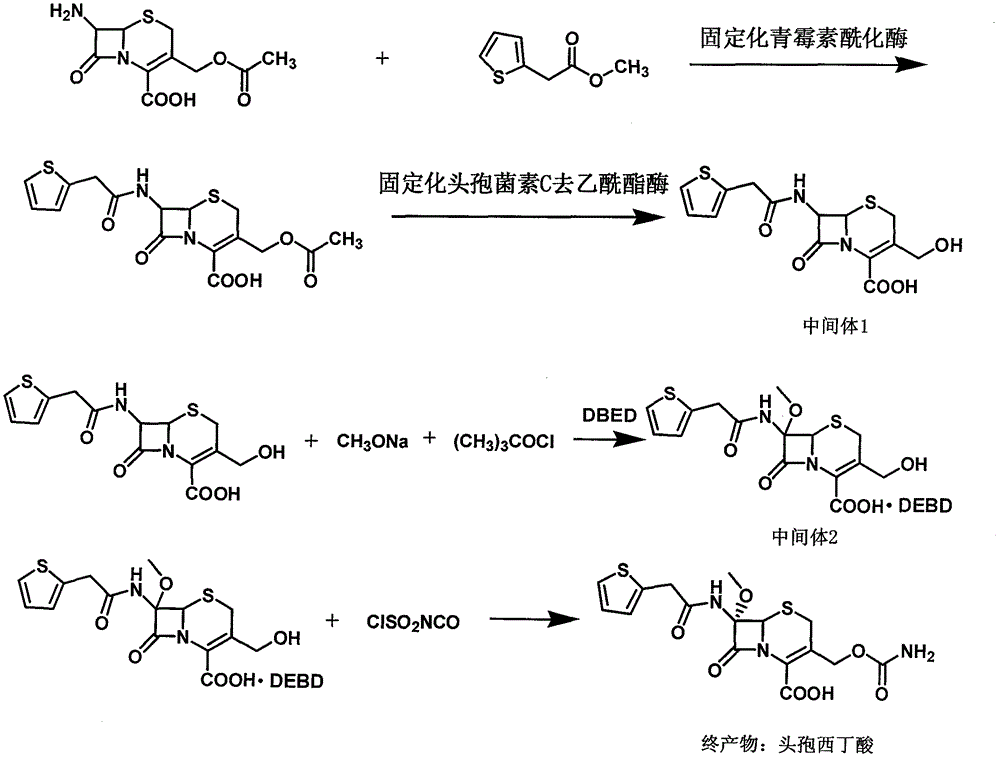
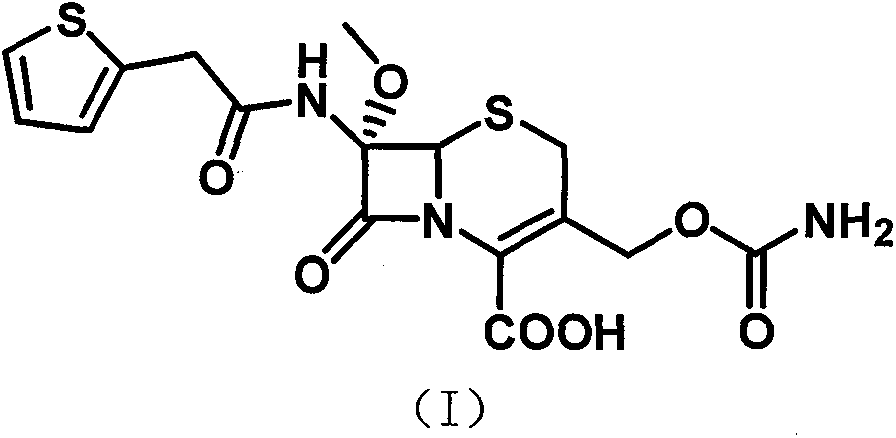
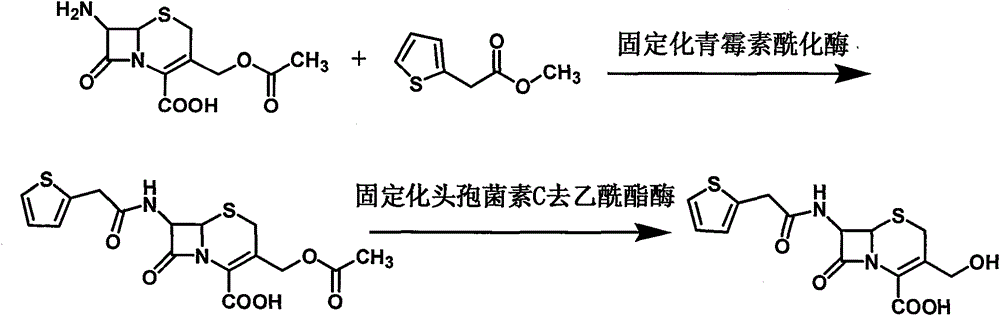
[0043] The synthetic method of cefoxitin acid of the present invention comprises the following steps:
[0044] 1) Dissolve 7-ACA in water, adjust the pH with ammonia water to dissolve 7-ACA, then add immobilized penicillin acylase, add methyl 2-thiopheneacetate dropwise, filter the filtrate after the reaction is complete, and add immobilized penicillin acylase Cephalosporin C deacetylesterase continues to react. After the reaction, the filtrate is filtered out, added with activated carbon for decolorization, filtered, then adjusted to pH and crystallized, filtered, and dried to obtain the intermediate 3-deacetyl cephalothinic acid.
[0045] 2) Dissolve the intermediate 3-deacetyl cephalothinic acid obtained in step 1) with a mixed solvent of dichloromethane, methanol and tetrahydrofuran, drop the sodium methoxide solution prepared in advance and cool down after dropping to a certain temperature, and finish the dripping Finally, add tert-butyl hypochlorite dropwise, then keep w...
Embodiment 1
[0047] The synthesis of embodiment 1,3-deacetyl cephalothinic acid
[0048] Put 80 grams of 7-ACA into a stirred flask, add 1000 ml of deionized water, start stirring and adjust the pH with 8% ammonia water to dissolve 7-ACA, control the temperature at 20°C, and put 30 grams of immobilized penicillin into acylation Enzyme, then add 55 grams of methyl 2-thiopheneacetate dropwise, and simultaneously use 8% ammonia water to control the pH6.5~7.0 until the pH no longer changes, and the liquid chromatography detects that the 7-ACA residue is less than 1.0%. Wash the immobilized enzyme with deionized water; add the filtrate to a stirred flask, control the temperature at 20°C, put in 20 grams of immobilized cephalosporin C deacetylesterase to start the reaction, and use 8% ammonia water to control the pH from 7.0 to 7.5 to pH Keep it unchanged for 3 minutes, then the reaction is over, filter and collect the filtrate, add 5 grams of activated carbon to the filtrate and stir to decolor...
Embodiment 2
[0049] Embodiment 2, the synthesis of 7-alpha-methoxy-3-deacetyl cephalothinic acid benzathine salt
[0050] Put 100 grams of 3-deacetyl cephalothinic acid, 1000 ml of dichloromethane, 100 ml of methanol, and 100 ml of tetrahydrofuran into a stirred flask, stir to dissolve, cool down to -85°C with liquid nitrogen, and then add dropwise to prepare in advance Sodium methoxide solution (90 grams of sodium methoxide + 200 milliliters of dichloromethane + 200 milliliters of methanol), control the temperature -83 ~ -87 ° C, dropwise add 35 grams of tert-butyl hypochlorite, and continue the reaction for 1 After 1 hour, add 100 ml of 50% glacial acetic acid solution, stir for 15 minutes and heat up to -50°C, then add 300 ml of 5% sodium chloride solution, heat up to 0-5°C, stir for 10 minutes and let stand to separate layers, the water phase Extract once with 100 ml of dichloromethane, combine the organic phases, add activated carbon for decolorization, filter, add 500 ml of water to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com