A kind of 3,3'-methylene-bisfluoroquinolone derivative containing ethyl quinoline ring and its preparation method and application
A technology of ethyl quinoline ring and bisfluoroquinolone, applied in the preparation of 3,3'-methylene-bisfluoroquinolone derivatives, in the field of 3,3'-methylene-bisfluoroquinolone derivatives, capable of Solve the problems of high toxicity of anti-tumor drugs, poor patient tolerance, and low cure rate of tumor diseases, and achieve the effect of increasing anti-tumor activity and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
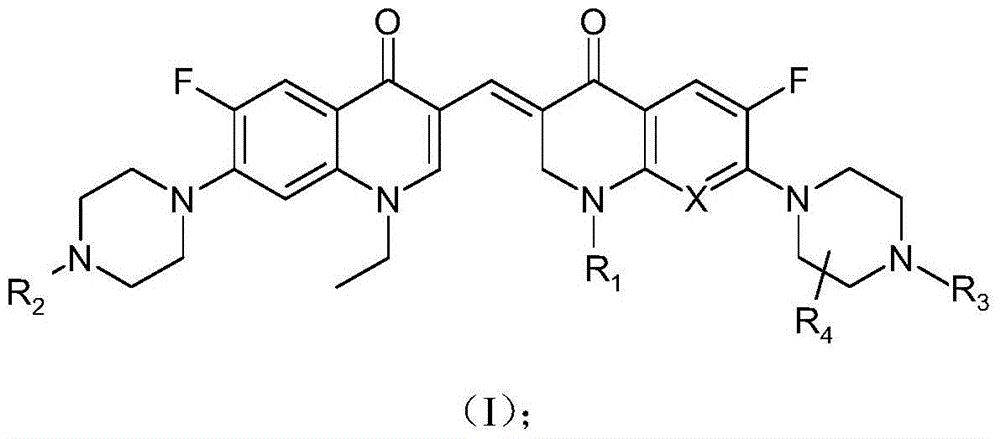
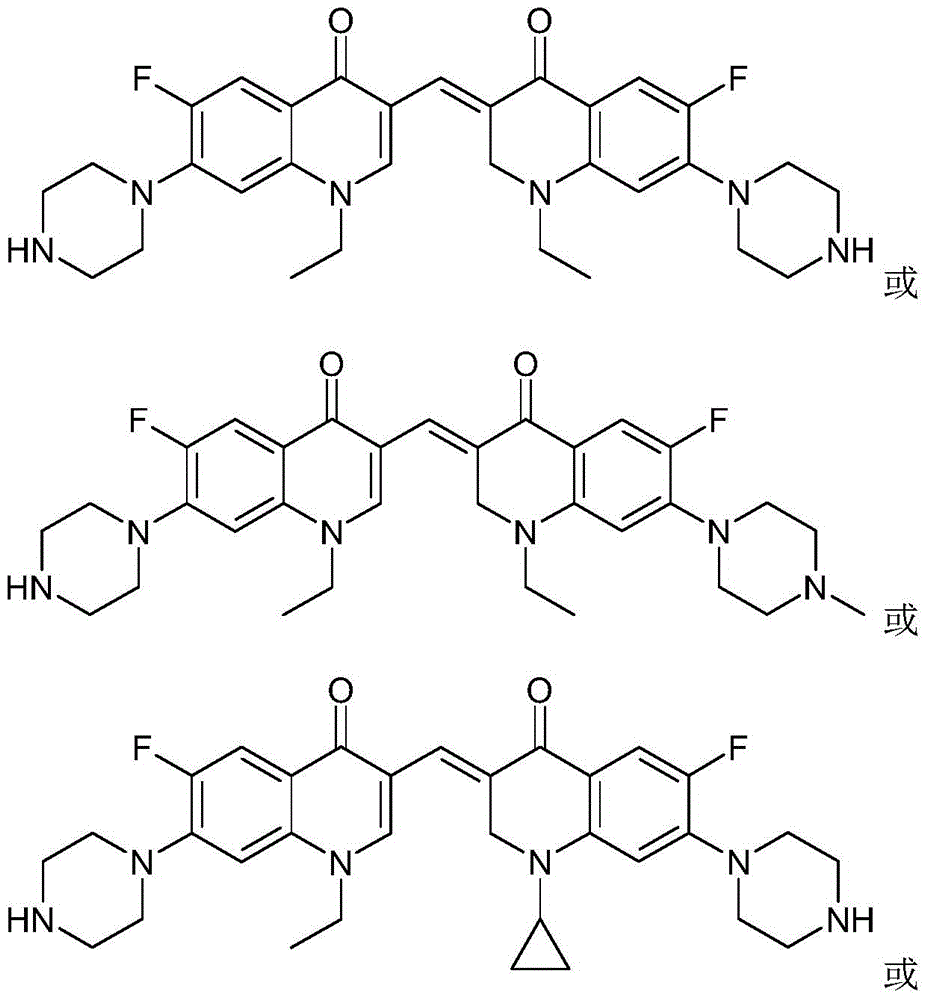
[0044] The 3,3'-methylene-bisfluoroquinolone derivative containing an ethylquinoline ring in this example is 1-ethyl-6-fluoro-7-piperazin-1-yl-3-[1-ethyl Base-6-fluoro-7-piperazin-1-yl-2,3-dihydro-quinolin-4(1H)-one-3-ylidenemethyl]quinolin-4(1H)-one, its chemical The structural formula is:
[0045]
[0046] That is, R in formula (I) 1 is ethyl, R 2 , R 3 and R 4 are hydrogen atoms, and X is a hydrocarbon group.
[0047] The preparation method of the 3,3'-methylene-bisfluoroquinolone derivatives containing an ethylquinoline ring in this embodiment is: take 0.5g (1.8mmol) of 1-ethyl-6-fluoro-7-piper Azin-1-yl-2,3-dihydro-quinolin-4(1H)-one and 0.55g (1.8mmol) of 1-ethyl-6-fluoro-7-piperazin-1-yl-quinoline -4(1H)-keto-3-carbaldehyde, dissolved in 20ml of absolute ethanol, 0.5ml of piperidine was added dropwise, after reflux reaction for 24h, left overnight, the resulting solid was collected by filtration, and recrystallized with DMF-ethanol to obtain light Yellow cryst...
Embodiment 2
[0049]The 3,3'-methylene-bisfluoroquinolone derivative containing an ethylquinoline ring in this example is 1-ethyl-6-fluoro-7-piperazin-1-yl-3-[1-ethyl Base-6-fluoro-7-(4-methylpiperazin-1-yl)-2,3-dihydro-quinolin-4(1H)-one-3-ylidenemethyl]quinoline-4(1H )-ketone, its chemical structural formula is:
[0050]
[0051] That is, R in formula (I) 1 is ethyl, R 2 is a hydrogen atom, R 3 is methyl, R 4 is a hydrogen atom, and X is a hydrocarbon group.
[0052] The preparation method of the 3,3'-methylene-bisfluoroquinolone derivative containing ethylquinoline ring of the present embodiment is: get 0.52g (1.8mmol) of 1-ethyl-6-fluoro-7-( 4-methylpiperazin-1-yl)-2,3-dihydro-quinolin-4(1H)-one with 0.55g (1.8mmol) of 1-ethyl-6-fluoro-7-piperazine- 1-yl-quinolin-4(1H)-one-3-carbaldehyde, dissolved in 20ml of absolute ethanol, added dropwise with 0.5ml of piperidine, after reflux reaction for 24h, the resulting solid was collected by filtration and recrystallized with DMF-ethan...
Embodiment 3
[0054] The 3,3'-methylene-bisfluoroquinolone derivative containing ethylquinoline ring in this example is 1-ethyl-6-fluoro-7-piperazin-1-yl-3-[1-ring Propan-6-fluoro-7-piperazin-1-yl-2,3-dihydro-quinolin-4(1H)-one-3-ylidenemethyl]quinolin-4(1H)-one, its chemical The structural formula is:
[0055]
[0056] That is, R in formula (I) 1 is cyclopropyl, R 2 , R 3 and R 4 are hydrogen atoms, and X is a hydrocarbon group.
[0057] The preparation method of the 3,3'-methylene-bisfluoroquinolone derivatives containing ethylquinoline ring in this example is: take 0.52g (1.8mmol) of 1-cyclopropyl-6-fluoro-7- Piperazin-1-yl-2,3-dihydro-quinolin-4(1H)-one with 0.55 g (1.8 mmol) of 1-ethyl-6-fluoro-7-piperazin-1-yl-quinone Phenyl-4(1H)-one-3-carbaldehyde was dissolved in 20ml of absolute ethanol, 0.5ml of piperidine was added dropwise, after reflux reaction for 24h, left overnight, the resulting solid was collected by filtration, and recrystallized with DMF-ethanol to obtain Ligh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com