Alpha-cyano-4-hydroxy cinnamic acid derivative and preparation method and application thereof
A technology of hydroxycinnamic acid and derivatives, which is applied in the field of pharmaceutical compounds and their preparation, can solve the problems of death, large toxic and side effects, inaccurate curative effect and the like, and achieves the effects of simple preparation process and excellent curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
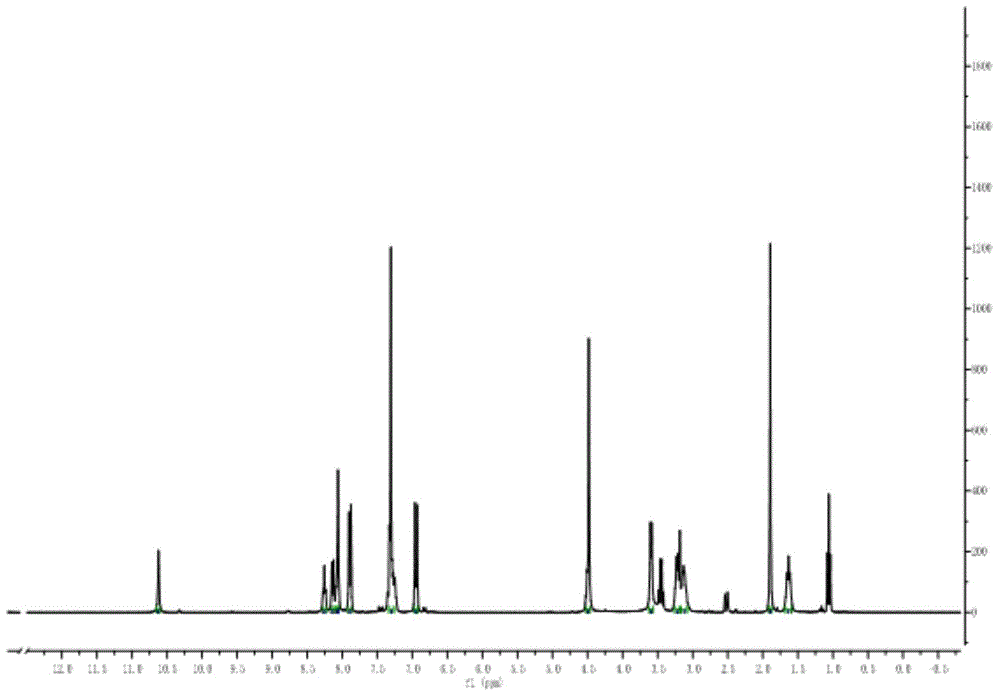
[0044] Preparation of α-cyano-4-hydroxycinnamic acid derivatives with aldose reductase inhibitory activity
[0045] 1.1.2-N-(N-acetyl-O-benzyl-D-seryl)-α-cyano-4-hydroxycinnamoylethylenediamide preparation
[0046] Include the following four steps.
[0047] (1) Preparation of 2-N-tert-butoxyacyl-α-cyano-4-hydroxycinnamoylethylenediamine: Add 946.0mg (5.0mmol ), add an appropriate amount of DCM / DMF=10:1 (V / V) and stir to dissolve, add 1.15g (6.0mmol) of EDC. mg (6.0 mmol) was added to the reaction flask, and the reaction was stirred at 0°C for 10 min to fully activate the free carboxyl groups. After the carboxyl group is fully activated, measure 0.9mL (5.5mmol) of N-Boc-ethylenediamine and 2.3mL (13.2mmol) of DIPEA into the reaction flask in turn, stir and react at 0°C for about 30min, then slowly rise to room temperature, and continue the reaction 24h. After the reaction was completed, the solvent was removed, and silica gel column chromatography (developing solvent: ethyl...
Embodiment 2
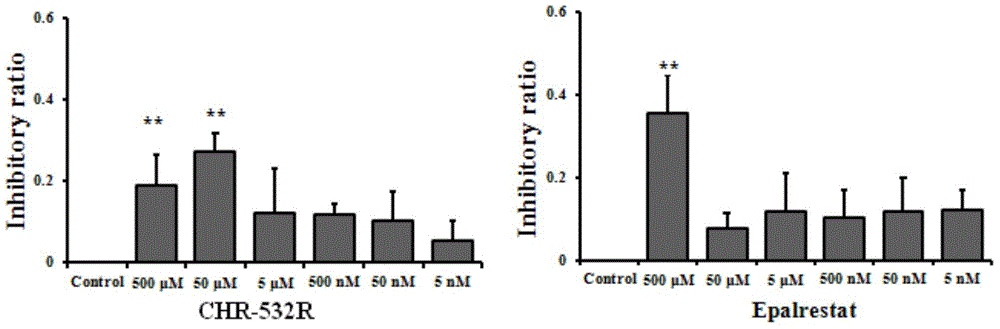
[0081] The synthetic compound of embodiment 1 is to the inhibitory activity of aldose reductase enzyme
[0082] In this example, aldose reductase (AR), NADPH, and DL-glyceraldehyde were all purchased from Wako Pure Chemical Industries, Ltd.; Epalrestat (EPS) was purchased from Tokyo Chemical Industry Co., Ltd.
[0083] Experimental procedure: Add 500 μL of pH 6.2 buffer solution, 100 μL of 1.5 mmol / L NADPH solution and 100 μL of 100 mmol / L DL-glyceraldehyde solution into a 48-well plate, then add 100 μL of sample solutions of different concentrations and 195 μL of distilled water. At the same time, a blank vehicle group (500 μL of pH 6.2 buffer solution, 100 μL of 1.5 mmol / L NADPH solution, 100 μL of 1 / 1000 solvent, 300 μL of distilled water), and an enzyme power group (500 μL of pH 6.2 buffer solution, 100 μL of 1.5 mmol / L NADPH solution, 100 mmol / L L DL-glyceraldehyde solution 100μL, 1 / 1000 vehicle 100μL, distilled water 195μL) and positive drug EPS group (PH6.2 buffer solut...
Embodiment 3
[0095] The in vitro antioxidant activity of the synthetic compound of embodiment 1
[0096] In this example, Trolox (6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) and Fluorescein (FL) (sodium salt) were purchased from Aldrich Company (Milwaukee, WI); 2,2' -Azobis (2-amidino-propane) dihydrochloride (AAPH) was purchased from Wako Pure Chemical Industries, Ltd., Japan.
[0097] Experimental steps:
[0098] Add 20 μL of pH 6.8 phosphate buffer to the 96-well plate, then add 20 μL of samples to be tested at different concentrations, measure 3 wells in parallel for each concentration sample, then add 140 μL of AAPH solution, and set up AAPH absorption holes (40 μL of phosphate buffer, AAPH solution 140 μL), fluorescence control wells (phosphate buffer 180 μL) and Trolox control wells (Trolox standard solution 20 μL, phosphate buffer 20 μL, AAPH solution 140 μL); after adding 20 μl of disodium fluorescein solution to each well to start the reaction, the 96 The orifice pl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com