Method and device for preparing hydrogen by electrolyzing ammonia
A technology for ammonia preparation and electrolysis of ammonia, applied in the direction of electrolysis process, electrolysis components, electrodes, etc., to achieve the effect of low energy consumption, low energy consumption and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of cathode and anode:
[0031] Dip the clean foam nickel electrode into the solution containing 5mmol / L H 2 PtCl 6 In the 0.5mol / L HCl solution, use the i-t curve method to deposit Pt at -0.3V, and the deposition time is 600s-3600s, that is, 0.5mg / cm 2 -2mg / cm 2 Platinum deposited amount of nickel foam cathode electrode.
[0032] Dip the clean nickel foam electrode into the 2 PtCl 6 and IrCl 3 In the 0.5mol / L HCl solution, where the mass ratio of Pt to Ir is 7:1, use the i-t curve method to deposit Pt at -0.3V, and the deposition time is 600s-3600s, that is, 0.5mg / cm 2 -2mg / cm 2 Nickel foam anode electrode deposited by double noble metal Pt / Ir.
[0033] Special device for the method of electrolyzing ammonia to produce hydrogen:
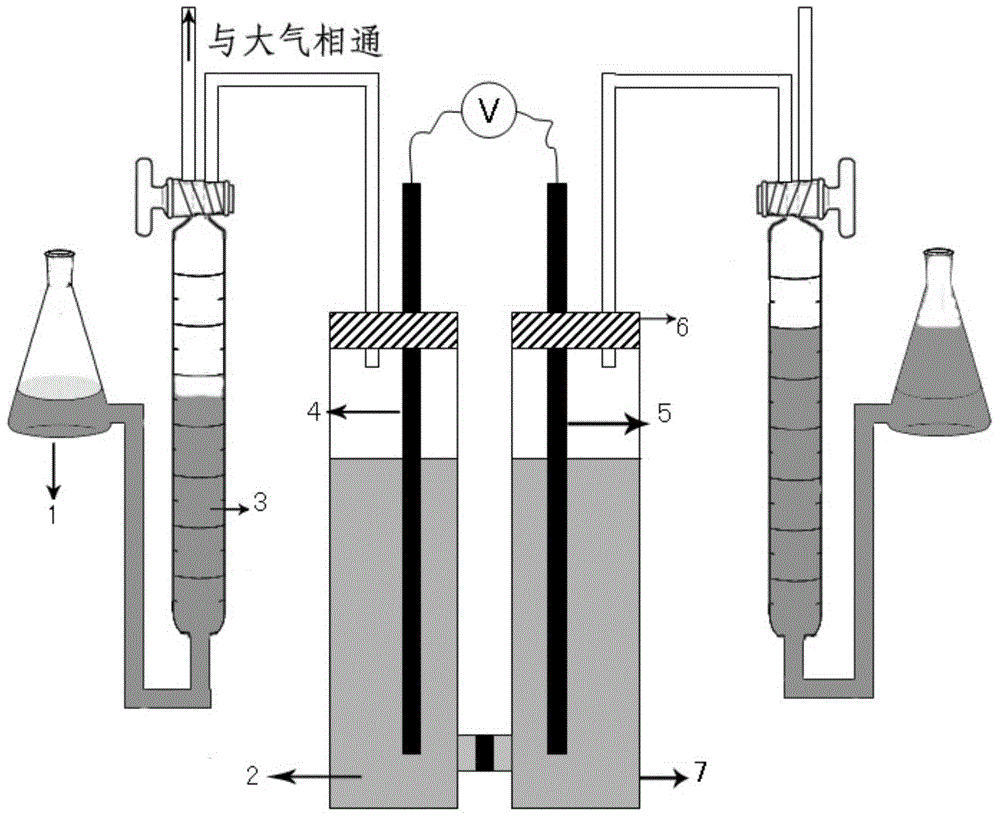
[0034] Such as figure 1 As shown, the special device is an H-type electrolytic cell. The horizontal side of the H-type electrolytic cell is provided with a glass sand disc, and the two vertical sides are respectively indep...
Embodiment 2
[0036] Hydrogen production by electrolysis of ammonia: Put 100mL of electrolyte solution composed of 2mol / L ammonia water, 5mol / L potassium hydroxide and water into the H-type electrolytic cell.
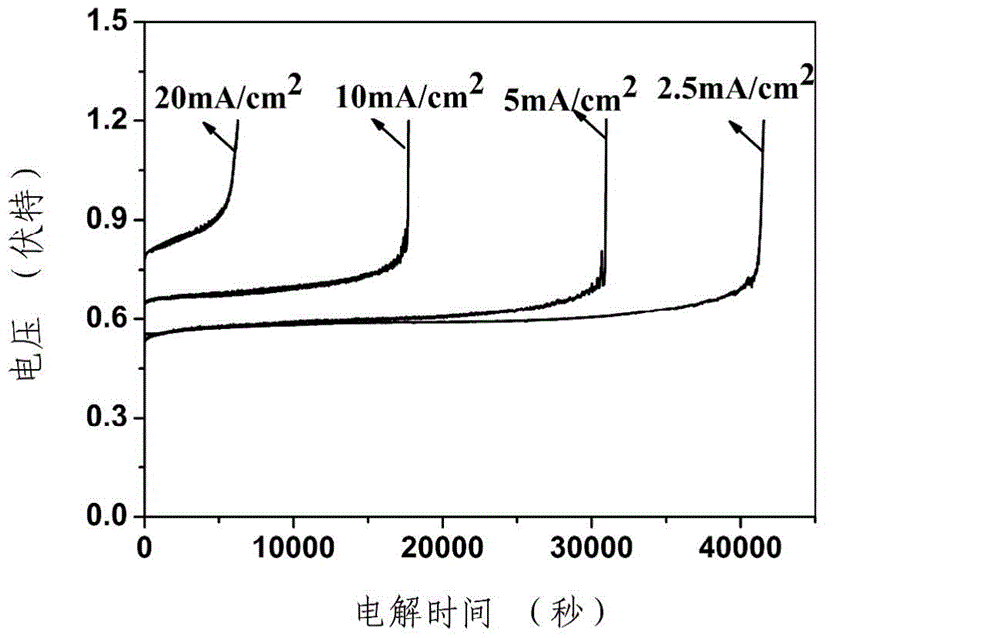
[0037] The electrolytic cell is divided into an anode chamber and a cathode chamber with a glass sand disc, wherein the anode electrode and the cathode electrode prepared by the above-mentioned embodiment are placed respectively; between the anode electrode and the cathode electrode, 2.5mA / cm 2The current, the obtained electrolysis voltage is about 0.6V, and the electrolysis voltage remains stable within 40000s. The hydrogen production rate is about 0.09mL / min.
Embodiment 3
[0039] Hydrogen production by electrolysis of ammonia: Put 100mL of electrolyte solution composed of 2mol / L ammonia water, 5mol / L potassium hydroxide and water into the H-type electrolytic cell.
[0040] The electrolytic cell is divided into an anode chamber and a cathode chamber with a glass sand disc, wherein an anode electrode and a cathode electrode are placed respectively; a 5mA / cm 2 The current, the obtained electrolysis voltage is about 0.68V, and the electrolysis voltage remains stable within 30000s. The hydrogen production rate is about 0.2mL / min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com