Organic blue light electrophosphorescent metal iridium complex, preparation method thereof and organic electroluminescent device
A technology of iridium metal complexes and phosphorescent materials, which is applied in the field of organic electroluminescent materials, can solve problems such as poor blue light color purity, and achieve the effects of reducing self-quenching, increasing solubility, and facilitating evaporation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
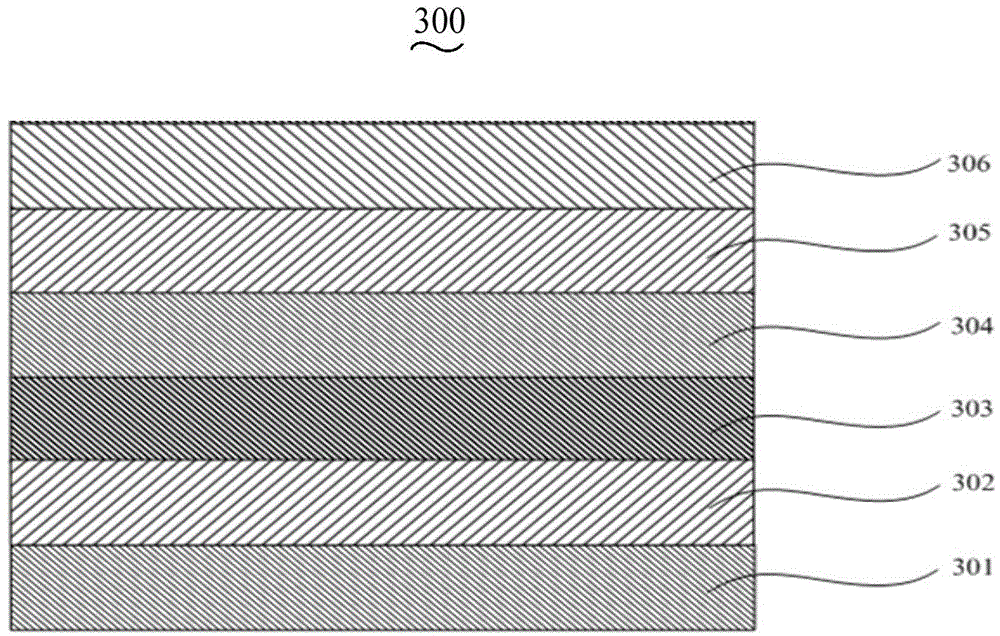
Image
Examples
preparation example Construction
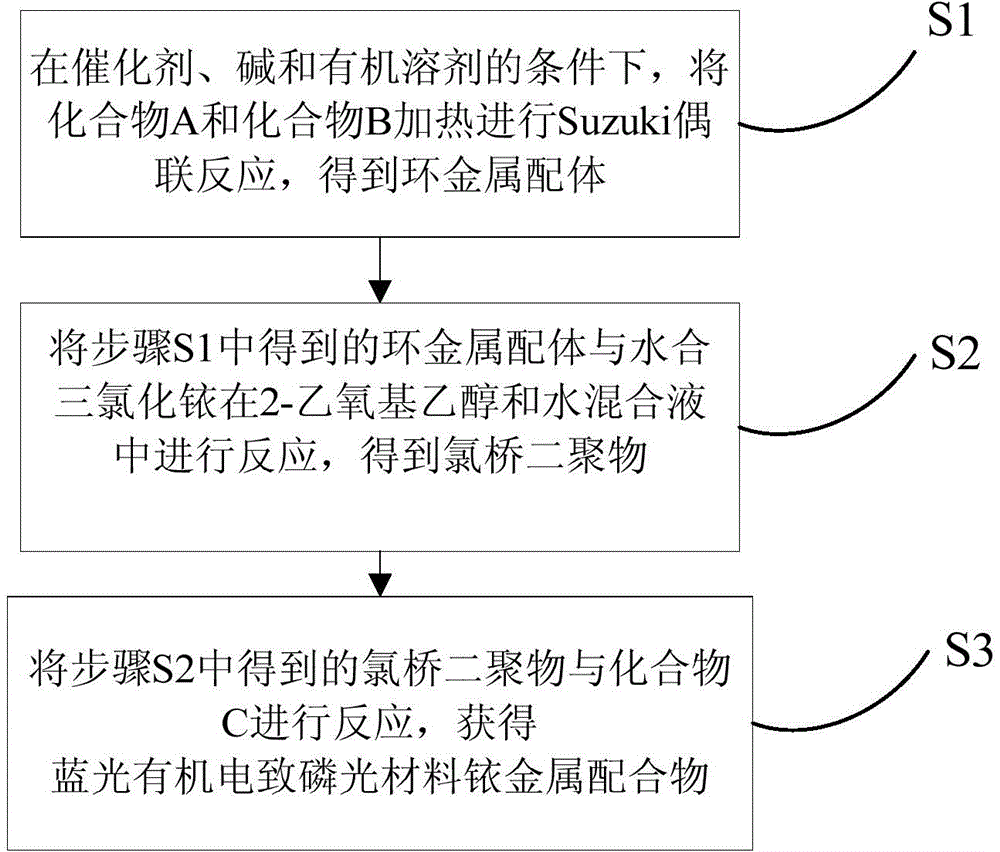
[0038] see figure 1 , the preparation method of the above-mentioned blue light organic electrophosphorescent material iridium metal complex, comprising the following steps:
[0039] S1, under the protection of inert gas, the structural formula is The compound A and the structural formula are The compound B is dissolved in the first organic solvent containing catalyst and base to obtain a reaction solution, the reaction solution is subjected to Suzuki coupling reaction at a temperature of 85-100° C. for 6-12 hours, and after the reaction is stopped, the reaction solution is separated and purified, get the structural formula as ring metal ligand; wherein, the molar ratio of compound A to compound B is 1:1 to 1:1.5; R is a hydrogen atom, C 1 ~C 20 Alkyl or C 1 ~C 20 The alkoxy group; the reaction formula is:
[0040]
[0041] S2. Under the protection of an inert gas, dissolve the cyclometal ligand and iridium trichloride trihydrate in a mixed solvent formed by 2-etho...
Embodiment 1
[0070] Example 1: Complex bis(2-(3',5'-difluoro-4'-trifluoromethylphenyl)pyrimidine-N,C 2 Synthesis of ')(3-trifluoromethyl-5-(2'-pyridyl)-1,2,4-triazole)iridium
[0071] (1) Synthesis of 2-(3',5'-difluoro-4'-trifluoromethylphenyl)pyrimidine
[0072]
[0073] Under nitrogen protection, 1.59g (10mmol) 2-bromopyrimidine (A1), 2.71g (12mmol) 3,5-difluoro-4-trifluoromethylphenylboronic acid (B) and 0.58g (0.5mmol) tetrakis ( Triphenylphosphine) palladium was dissolved in 40mL of toluene and stirred for 10min. Subsequently, 20 mL of an aqueous solution containing 2.76 g (20 mmol) of potassium carbonate was added dropwise to form a reaction liquid. Heated and stirred at 100°C for 6h. After the reaction solution was cooled to room temperature, it was extracted with dichloromethane, separated, washed with water until neutral, and dried over anhydrous magnesium sulfate. After filtration, the filtrate was distilled off the solvent under reduced pressure to obtain the crude produc...
Embodiment 2
[0092] Example 2: Complex bis(2-(3',5'-difluoro-4'-trifluoromethylphenyl)-5-methylpyrimidine-N,C 2 Synthesis of ')(3-trifluoromethyl-5-(2'-pyridyl)-1,2,4-triazole)iridium
[0093] (1) Synthesis of 2-(3',5'-difluoro-4'-trifluoromethylphenyl)-5-methylpyrimidine
[0094]
[0095] Under nitrogen protection, 1.73g (10mmol) 2-bromo-5-methylpyrimidine (A2), 2.48g (11mmol) B and 0.28g (0.4mmol) dichlorobis(triphenylphosphine)palladium were dissolved in 50mL In DMF, stir for 10 min. Subsequently, 25 mL of an aqueous solution containing 3.18 g (30 mmol) of sodium carbonate was added dropwise to the reaction system to form a reaction liquid. Stir the reaction under heating to 90°C for 8 hours. After the reaction solution was cooled to room temperature, it was extracted with dichloromethane, separated, washed with water until neutral, and dried over anhydrous magnesium sulfate. After filtration, the filtrate was distilled off the solvent under reduced pressure to obtain the crude p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com