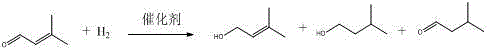Method for synthesizing prenol employing selective hydrogenation of 3-methylcrotonaldehyde
A technology of prenyl aldehyde and prenol, applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve problems such as undescribed examples of prenyl aldehyde hydrogenation, and achieve easy operation , Reduce production cost, low unit consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 6g of ruthenium chloride and tris-(p-phenoxy-polyoxyethylene ether)-phosphine complex catalyst, 500g of water into a 2L autoclave with magnetic stirring and temperature controller, and then add 200g of prenaldehyde . The mass ratio of ruthenium chloride to tris-(p-phenoxy-polyoxyethylene ether)-phosphine in the complex catalyst is 0.009:1.
[0042] It was replaced three times with nitrogen, and then three times with hydrogen.
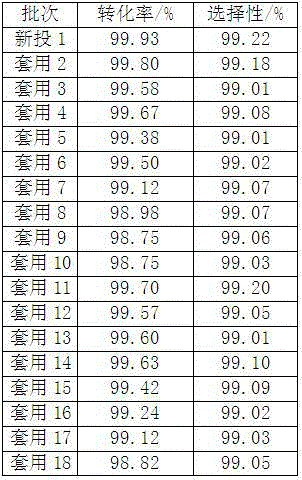
[0043] Heating to 150°C, pressurizing hydrogen to 3.0MPa, stirring at 700rpm, reacting for 3 hours, sampling and analyzing the conversion rate of prenaldehyde is greater than 99%.
[0044] Cool with water, press out the reaction liquid, let it stand for stratification, the water phase containing the catalyst can be applied to the next batch of reactions, and the organic phase is prenol.
[0045] According to gas chromatography analysis, the conversion rate of prenaldehyde was 99.98%, and the selectivity of prenol was 99.10%.
Embodiment 2
[0047] Add 25g of rhodium nitrate and sodium triphenylphosphinesulfonate complex catalyst, 200g of water into a 2L autoclave with magnetic stirring and temperature controller, and then add 300g of prenaldehyde. The mass ratio of complex catalyst rhodium nitrate to sodium triphenylphosphine sulfonate is 0.001:1.
[0048] It was replaced three times with nitrogen, and then three times with hydrogen.
[0049] Heating to 30°C, pressurizing hydrogen to 5.0MPa, stirring at 700rpm, reacting for 28 hours, sampling and analyzing the conversion rate of prenaldehyde is greater than 98%.
[0050] Cool with water, press out the reaction liquid, let it stand for stratification, the water phase containing the catalyst can be applied to the next batch of reactions, and the organic phase is prenol.
[0051] According to gas chromatography analysis, the conversion rate of prenaldehyde was 98.15%, and the selectivity of prenol was 99.20%.
Embodiment 3
[0053] Add 25g of rhodium nitrate and tris-(p-phenoxy-polyoxyethylene ether)-phosphine complex catalyst, 800g of water into a 2L autoclave with magnetic stirring and temperature controller, and then add 100g of prenaldehyde. The mass ratio of rhodium nitrate to tris-(p-phenoxy-polyoxyethylene ether)-phosphine in the complex catalyst is 0.01:1.
[0054] It was replaced three times with nitrogen, and then three times with hydrogen.
[0055] Heating to 100°C, pressurizing hydrogen to 0.5MPa, stirring at 500rpm, reacting for 18.5 hours, sampling and analyzing the conversion rate of prenaldehyde is greater than 98%.
[0056] Cool with water, press out the reaction liquid, let it stand for stratification, the water phase containing the catalyst can be applied to the next batch of reactions, and the organic phase is prenol.
[0057] According to gas chromatography analysis, the conversion rate of prenaldehyde is 98.05%, and the selectivity of prenol is 99.40%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com