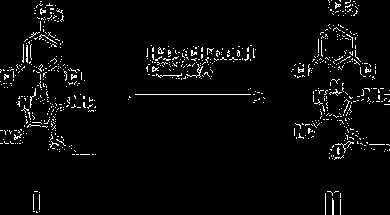
Process for preparing high-purity ethiprole through oxidation method
A technology of ethiprole and high purity, which is applied in the field of production and preparation of high-purity ethiprole, and can solve problems such as low yield, high cost, and complicated separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 10.287kg (27mol) of ethiprole oxidation precursor, 107.26kg (1083mol) of dichloroethane into a 100L reactor, add 4.212kg (70.2mol) of acetic acid and 6.8175kg (70.2mol) of 35% hydrogen peroxide, and stir at 30°C About 10 hours. Sampling and follow-up, after the reaction was completed, 3.285 kg (31.6 mol) of sodium sulfite was slowly added, and the exotherm was obvious, which was controlled within 55 °C. Add sodium hydroxide solution to adjust the pH to 6-7, then heat up to reflux (about 80°C), then cool to below 10°C, crystallize, and filter with suction to obtain 10.50kg of crude solid, with a purity of 98.7%, and a crude yield of 96%, containing The peroxide impurity is 0.5%. After one recrystallization, the fine product with peroxidic impurity less than 0.3% is obtained, and the fine product yield is 93%.
[0041] The purity of the synthetic ethiprole: 99.4% (HPLC).
Embodiment 2
[0043] Add 10.287kg (27mol) of ethiprole oxidation precursor, 107.26kg (1083mol) dichloroethane layer into a 100L reactor, add 1.62kg (27mol) of acetic acid and 6.8175kg (70.2mol) of 35% hydrogen peroxide, and stir at 30°C About 20 hours. Sampling and follow-up, after the reaction was completed, 3.285 kg (31.6 mol) of sodium sulfite was slowly added, and the exotherm was obvious, which was controlled within 55 °C. Add sodium hydroxide solution to adjust the pH to 6-7, then heat up to reflux (about 80°C), then cool to below 10°C, crystallize, and filter with suction to obtain 10.41kg of crude solid, with a purity of 98.3%, and a crude yield of 95%, containing The peroxide impurity is 0.6%. After one recrystallization, the fine product with peroxidic impurity less than 0.3% is obtained, and the fine product yield is 92%.
[0044] The purity of the synthetic ethiprole: 99.3% (HPLC).
Embodiment 3
[0046] Add 10.287kg (27mol) of ethiprole oxidation precursor, 107.26kg (1083mol) of dichloroethane layer into a 100L reactor, add 4.212kg (70.2mol) of acetic acid and 6.8175kg (70.2mol) of 35% hydrogen peroxide, 30°C Stir for about 18 hours. Sampling and follow-up, after the reaction was completed, 3.285 kg (31.6 mol) of sodium sulfite was slowly added, and the exotherm was obvious, which was controlled within 55 °C. Add sodium hydroxide solution to adjust the pH to 6-7, then heat up to reflux (about 80°C), then cool to below 10°C, crystallize, and filter with suction to obtain 10.40kg of crude solid, with a purity of 97.8%, and a crude yield of 95%, containing The peroxide impurity is 0.8%. After recrystallization twice, a fine product with peroxidic impurity less than 0.3% is obtained, and the fine product yield is 91%.
[0047] The purity of the synthetic ethiprole: 99.1% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com