A class of soluble polyaryletherketone containing diisopropylfluorene structure and its preparation method
A technology of polyaryletherketone and propylfluorene, which is applied in the field of high-performance polyaryletherketone polymers and their preparation, can solve the problems of limited varieties and achieve the effects of low cost, simple synthesis route and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
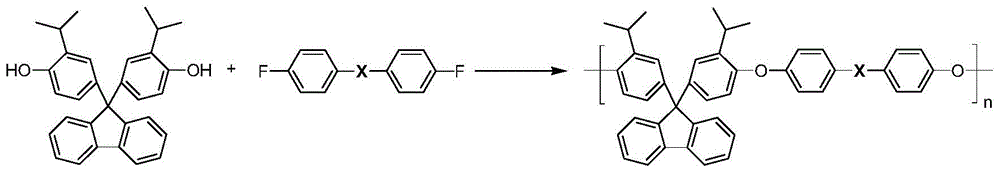
[0024] In a dry 50ml three-necked round-bottomed flask with nitrogen, add 2.6074g (6mmol) of bisphenol monomer containing diisopropylfluorene structure, 1.3092 (6mmol) active difluoroketone monomer (in the form of 4,4' -difluorobenzophenone as an example), 0.8293g (6mmol) of potassium carbonate, 15.3ml of N-methylpyrrolidone, 3.5ml of toluene, after azeotropic dehydration at 130°C for 3h, distill the toluene, and further heat up to After the polycondensation reaction at 160°C for 10 hours, the reaction was completed, and the reactant was poured into ethanol for sedimentation to obtain a fibrous polyaryletherketone polymer with a yield of 98% (based on the conversion of bisphenol monomers containing diisopropylfluorene structure rate meter).
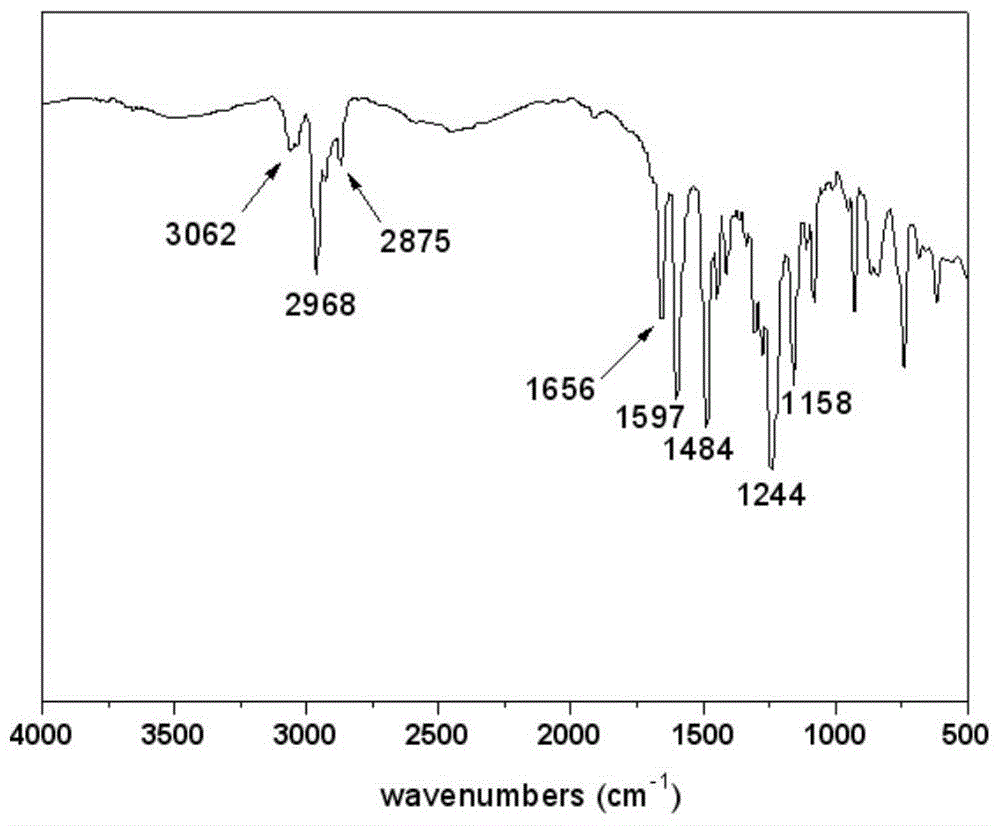
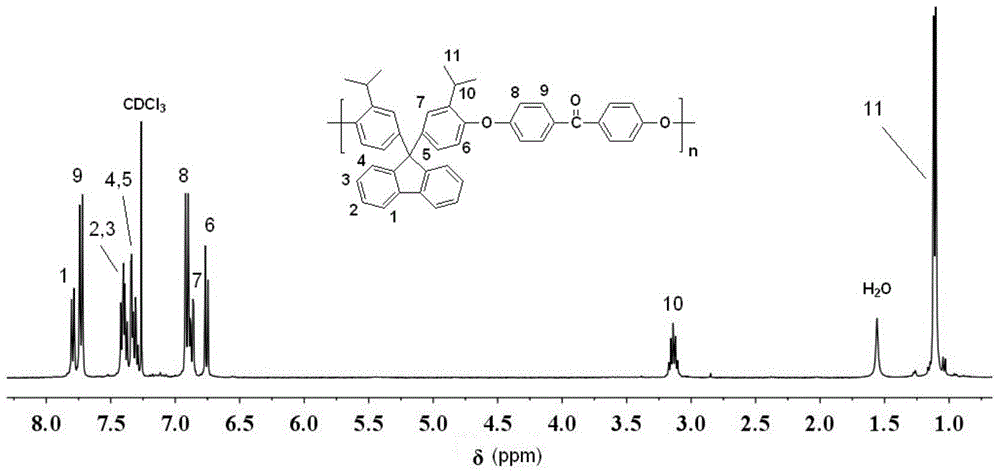
[0025] FT-IR(KBr)ν / cm -1 :3062,2968,2875,1656,1597,1484,1244,1158. 1 HNMR (CDCl 3 ,400MHz)δ:7.80(d,2H),7.73(d,4H),7.37-7.42(m,4H),7.29-7.34(m,4H),6.91(d,4H),6.87(m,2H) , 6.76(d,2H). Such as figure 1 and 2 shown.
Embodiment 2
[0027] In a dry 50ml three-necked round-bottomed flask with nitrogen, add 2.6074g (6mmol) of bisphenol monomer containing diisopropylfluorene structure, 1.3092 (6mmol) active difluoroketone monomer (in the form of 4,4' -difluorobenzophenone as an example), 2.0732g (15mmol) of potassium carbonate, 19.0ml of N-methylpyrrolidone, 7.6ml of toluene, after azeotropic dehydration at 150°C for 1h, distill the toluene, and further heat up to After the polycondensation reaction at 170°C for 6 hours, the reaction was terminated, and the reactants were poured into ethanol for sedimentation to obtain a fibrous polyaryletherketone polymer with a yield of 98% (based on the conversion of bisphenol monomers containing diisopropylfluorene structure rate meter).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com