Sterility test process isolator
A technology of isolator and process, applied in the direction of manufacturing tools, manipulators, chemistry, etc., to achieve the effect of reducing pollution, lowering the requirements of cleanliness level, and increasing controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
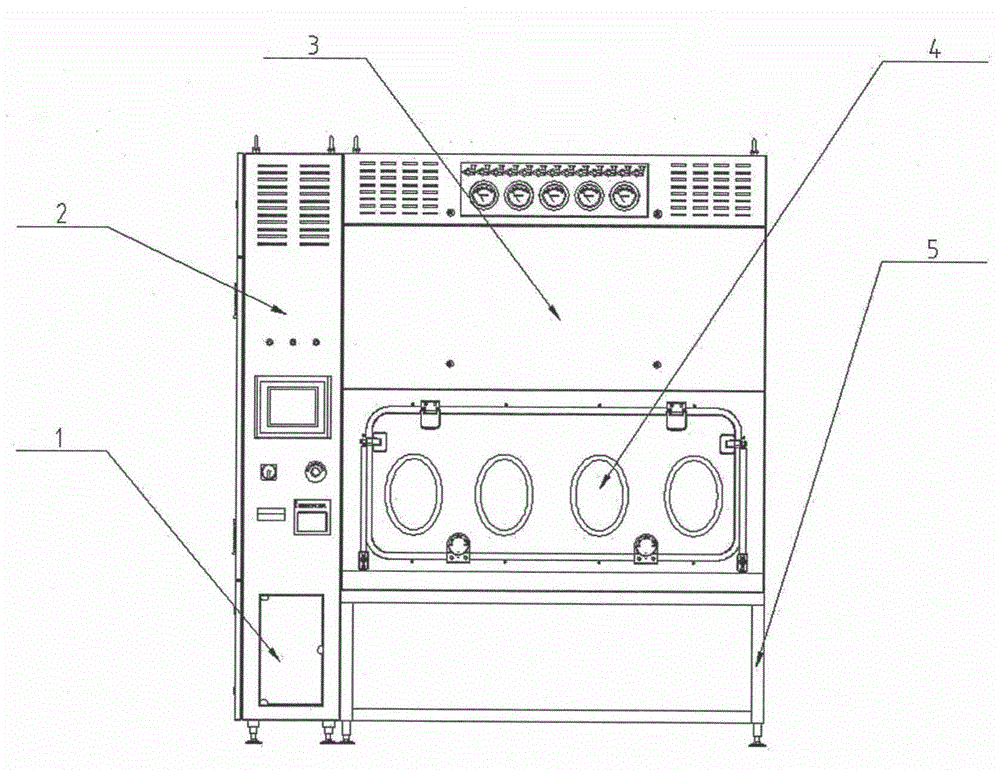
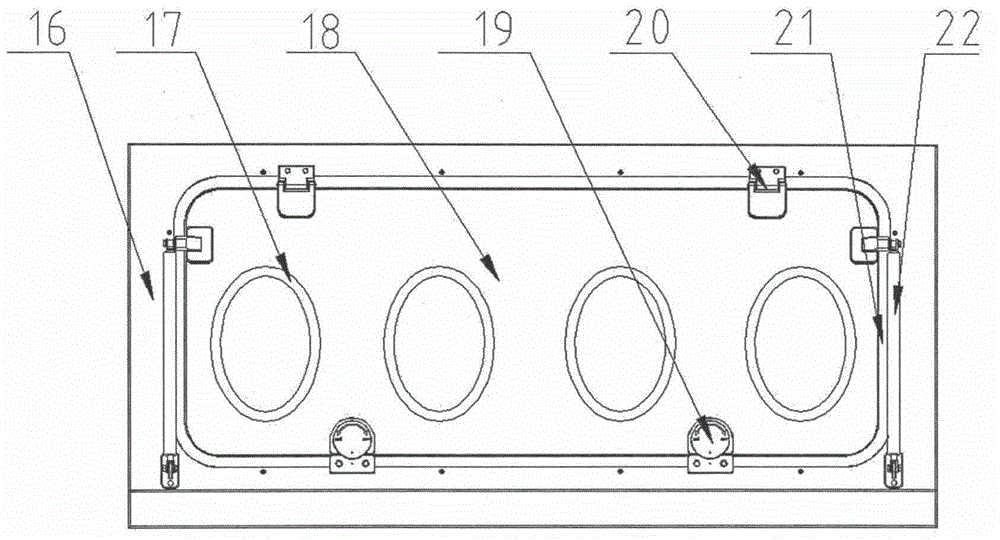
[0030] Such as figure 1 As shown, it is a schematic structural view of a sterile inspection process isolator provided by the present invention, including a frame assembly 5 at the bottom, a glove box assembly 4 arranged on the frame assembly 5, an air handling system 3 at the top, and an integrated Electrical system 2 of hydrogen peroxide vapor sterilization system 1.
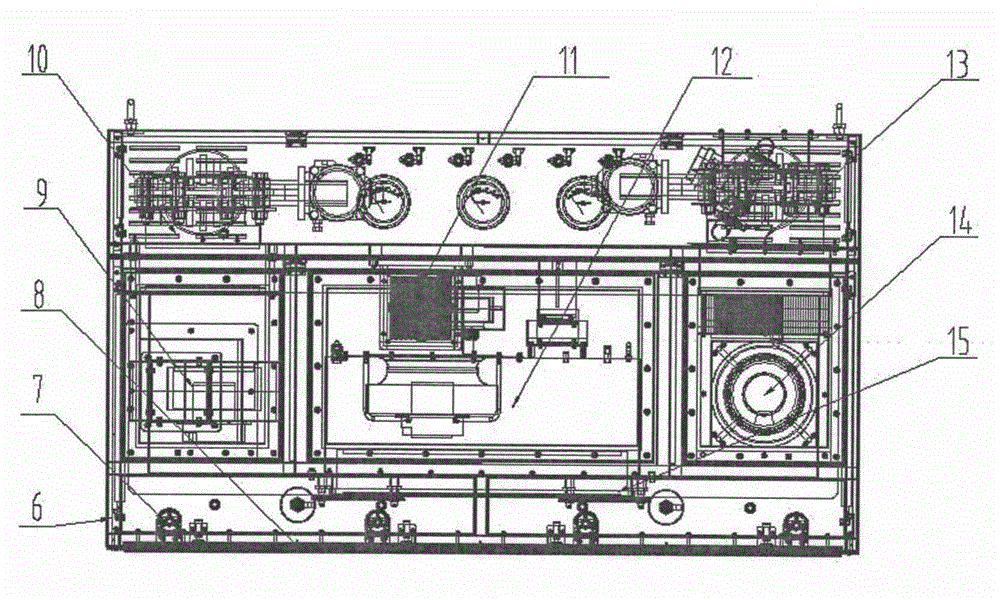
[0031] Such as figure 2 As shown, the air treatment system 3 includes the air treatment system mounting bracket 6 and the lighting lamp 7, the flow equalizing film 8, the fresh air assembly 9, the fresh air valve assembly 10, the internal circulation catalytic decomposition unit 11, Circulation fan assembly 12, exhaust valve assembly 13, exhaust assembly 14, liquid tank sealing filter 15, lighting lamp 7 are fixed on the mounting bracket through the lighting lamp mounting seat installed on the air treatment system bracket 6, and the flow equalizing membrane 8 , the fresh air assembly 9, the fresh air valve a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com