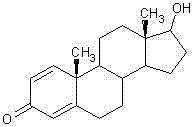
Method for preparing boldenone through selective reduction
A Boldenone and selectivity technology, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of prolonging the production cycle, increasing production costs, increasing reaction steps, etc., and achieve the effects of reducing production processes, low cost, and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 350ml of methanol and 100ml of water into the reaction flask, stir and cool down to -10°C, and add 4.5g of sodium borohydride. Then add 50 g of 1,4-androstenedione that has been crushed and passed through a 20-mesh sieve in portions at -10°C to -5°C, and the time for adding 1,4-androstenedione is 20 minutes to 30 minutes. After the addition is complete, continue to stir and react at -10°C to -5°C for 0.5 hours. The reaction solution was added to water cooled to 0°C-5°C in advance, stirred at 0°C-5°C for 0.5 hour, filtered with suction and dried to obtain 49.7 g of crude product. The crude product was then crystallized from a mixed solvent of methanol and ethyl acetate to obtain 47.6 g of Boldenone with an HPLC purity of 98.6%.
Embodiment 2
[0034] Add 350ml of methanol and 50ml of water into the reaction flask, stir and cool down to -10°C, and add 5.5g of potassium borohydride. Then add 50 g of 1,4-androstenedione that has been crushed and passed through a 20-mesh sieve in portions at -10°C to -5°C, and the time for adding 1,4-androstenedione is 20 minutes to 30 minutes. After the addition is complete, continue to stir and react at -10°C to -5°C for 45 minutes. The reaction solution was added to water cooled to 0°C-5°C in advance, stirred at 0°C-5°C for 0.5 hour, filtered with suction and dried to obtain 49.5 g of crude product. The crude product was crystallized from a mixed solvent of methanol and ethyl acetate to obtain 47.5 g of Boldenone, with an HPLC purity of 98.6%.
Embodiment 3
[0036]Add 350ml of methanol and 60ml of water into the reaction flask, stir and cool down to -10°C, and add 5g of potassium borohydride. Then add 50 g of 1,4-androstenedione that has been crushed and passed through a 20-mesh sieve in portions at -10°C to -5°C, and the time for adding 1,4-androstenedione is 20 minutes to 30 minutes. After the addition is complete, continue to stir and react at -10°C to -5°C for 45 minutes. The reaction solution was added to water cooled to 0°C-5°C in advance, stirred at 0°C-5°C for 0.5 hour, filtered with suction and dried to obtain 49.6 g of crude product. The crude product was then crystallized from a mixed solvent of methanol and ethyl acetate to obtain 47.6 g of boldenone, with an HPLC purity of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com