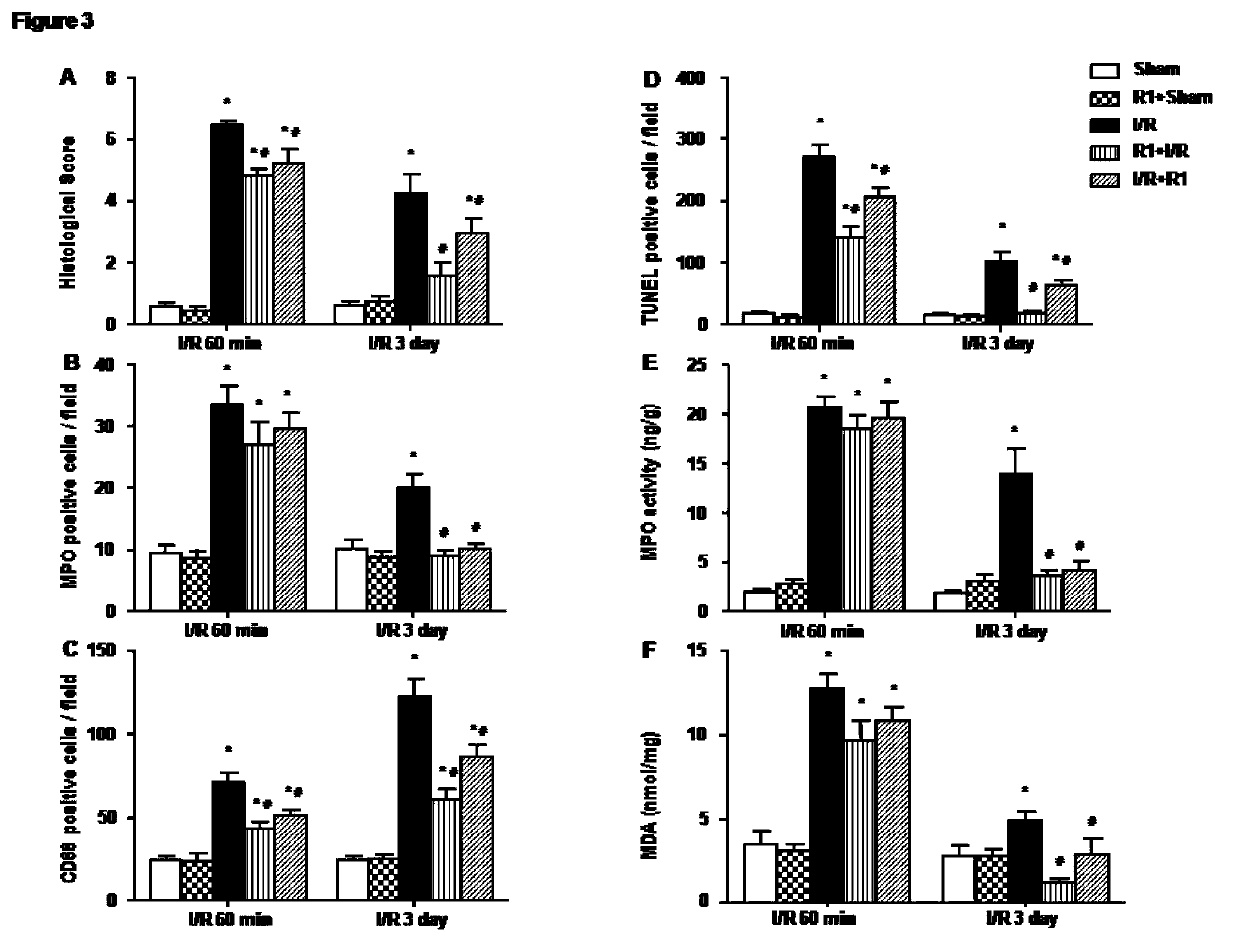
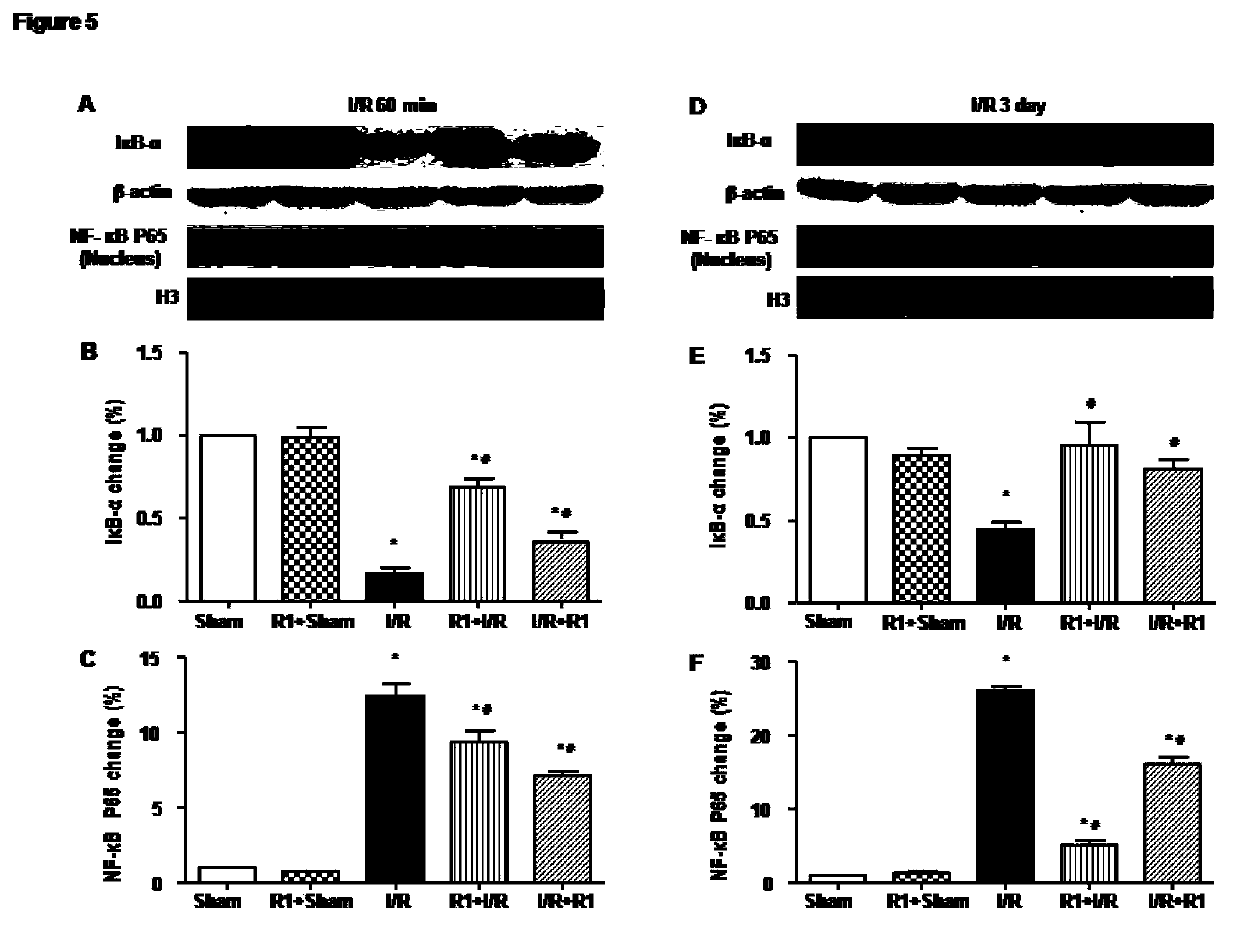
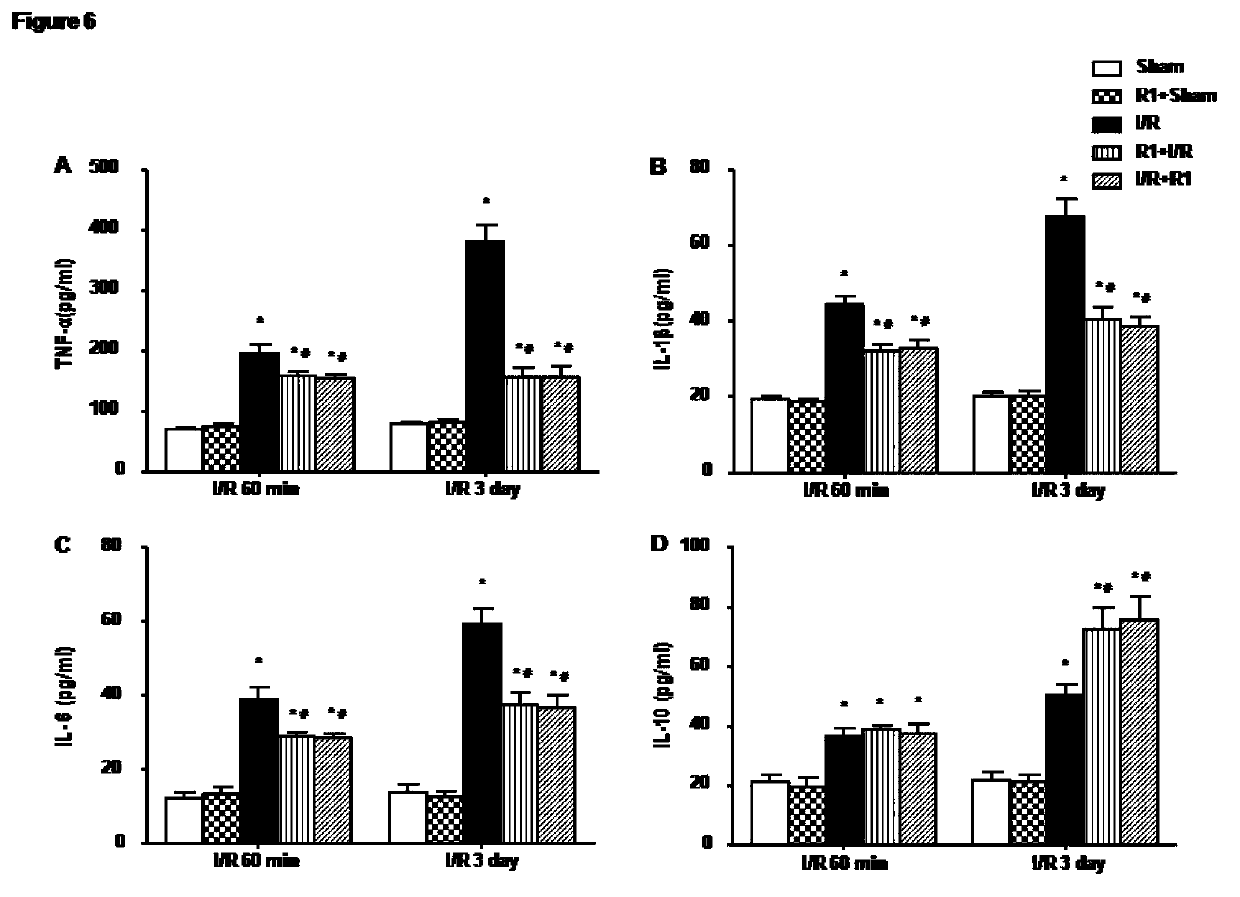
Protective effect of notoginsenoside R1 on small intestine ischemia/reperfusion injury
A technology of ischemia-reperfusion and notoginseng saponins, applied to medical preparations containing active ingredients, digestive system, organic active ingredients, etc., can solve the problem of no report on the protective effect of small intestine ischemia-reperfusion injury in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] tablet
[0147] 【prescription】
[0148] Notoginsenoside R1 100g
[0149] Microcrystalline cellulose 50g
[0150] Micronized silica gel 3g
[0151] Magnesium stearate 1.5g
[0152] [Preparation method] Take the original and auxiliary materials through a 100-mesh sieve; take the notoginsenoside R1 and microcrystalline cellulose, mix them, use 60% ethanol as a binder to make a soft material, and pass through a 20-mesh sieve to make granules, 60℃ Dry, take out, sieved through a 30-mesh sieve, add micro-powdered silica gel and magnesium stearate, mix well, and press to make 1000 tablets, which are ready to be obtained.
Embodiment 2
[0154] 【prescription】
[0155] Notoginsenoside R1 75g
[0156] Microcrystalline cellulose 37g
[0157] Micronized silica gel 2.3g
[0158] Magnesium stearate 1.1g
[0159] [Preparation method] Take the original and auxiliary materials through a 100-mesh sieve; take the notoginsenoside R1 and microcrystalline cellulose, mix them, use 60% ethanol as a binder to make a soft material, and pass through a 20-mesh sieve to make granules, 60℃ Dry, take out, sieving through a 30-mesh sieve, add appropriate amount of finely powdered silica gel and magnesium stearate, mix well, and press to make 1000 tablets, ready to be obtained.
Embodiment 3
[0161] 【prescription】
[0162] Notoginsenoside R1 133g
[0163] Microcrystalline cellulose 66g
[0164] Micronized silica gel 4g
[0165] Magnesium stearate 2g
[0166] [Preparation method] Take the original and auxiliary materials through a 100-mesh sieve; take the notoginsenoside R1 and microcrystalline cellulose, mix them, use 60% ethanol as a binder to make a soft material, and pass through a 20-mesh sieve to make granules, 60℃ Dry, take out, sieving through a 30-mesh sieve, add appropriate amount of finely powdered silica gel and magnesium stearate, mix well, and press to make 1000 tablets, ready to be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com