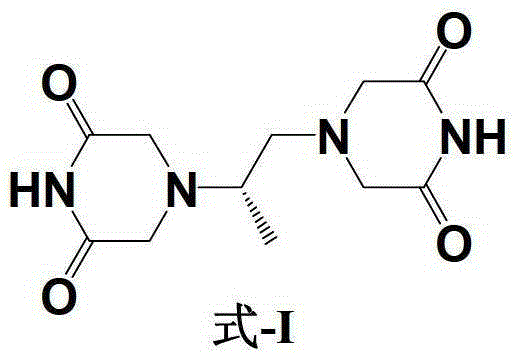
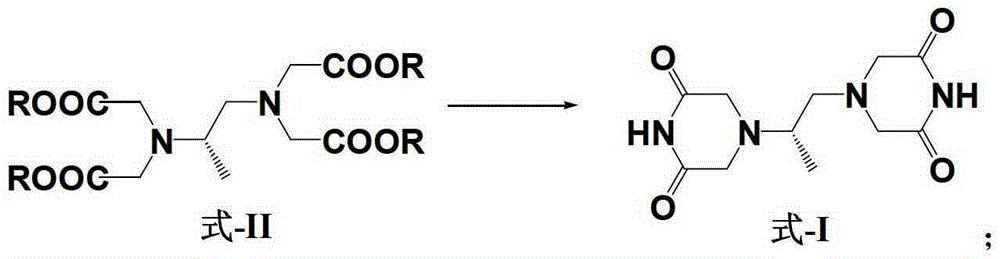
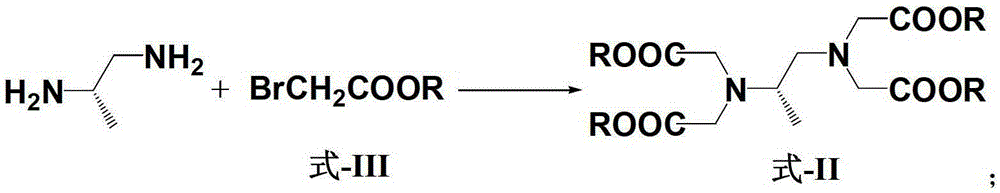
A kind of preparation method of dextropropanimine
A technology of dextropropyl imine and formamide, which is applied in the field of preparation of dextropropyl imine, can solve the problems of high cost, harsh reaction conditions, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: the preparation of (S)-1,2-diaminopropane-tetraacetic acid methyl ester:
Embodiment approach 11
[0080] (S)-1,2-diaminopropane hydrochloride (500mg, 3.4mmol, 1eq), methyl bromoacetate (5202mg, 34mmol, 10eq), potassium carbonate (4692mg, 34mmol, 10eq), acetonitrile (34mL, 0.1M) were added to 100mL single-necked bottles, and reacted at 25°C for 24 hours. After the reaction was completed, the inorganic salts were filtered off, the filtrate was concentrated to dryness, and HCl (15mL, 12%) solution was added to acidify, and petroleum ether (15ml×2, 60~90℃), adjusted the pH to 10 with saturated sodium carbonate solution, extracted with dichloromethane (15ml×3), dried and concentrated to dryness to obtain the crude product (S)-1,2-diaminopropane-tetra Methyl acetate, crude product yield 55%.
Embodiment approach 12
[0082] (S)-1,2-diaminopropane hydrochloride (500mg, 3.4mmol, 1eq), methyl bromoacetate (5202mg, 34mmol, 10eq), potassium carbonate (4692mg, 34mmol, 10eq), acetonitrile (34mL, 0.1M) were added to 100mL single-necked bottles, and reacted at 25°C for 48 hours. After the reaction was completed, the inorganic salts were filtered off, the filtrate was concentrated to dryness, acidified by adding HCl (15mL, 12%) solution, and petroleum ether (15ml×2, 60~90℃), adjusted the pH to 10 with saturated sodium carbonate solution, extracted with dichloromethane (15ml×3), dried and concentrated to dryness to obtain the crude product (S)-1,2-diaminopropane-tetra Methyl acetate, crude product yield 68%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com