Preparation method of 3,3',4,4'-tetraminodiphenyl sulfide
A technology of tetraamine diphenyl sulfide and dinitrobenzene, which is applied in the field of functional material technology and organic synthesis, and can solve the problems of many by-products, difficulty in industrialization, and complicated operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
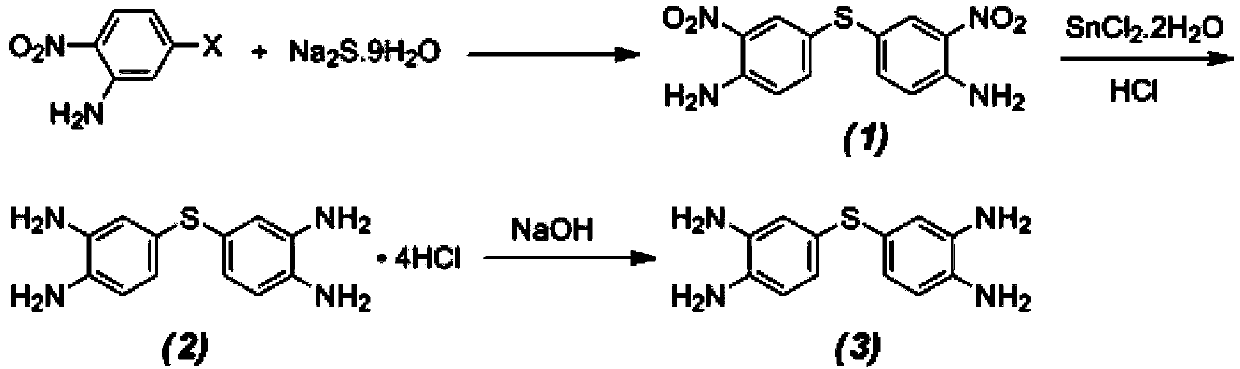
[0025] Step 1) Preparation of 4,4'-thiobis(2-nitroaniline)
[0026] Add 40mL of absolute ethanol and 2.7382g (0.0159mol) of 5-chloro-2-nitroaniline to a three-necked flask equipped with a thermometer, dropping funnel, and reflux condenser, stir well, and sulfide 4.85g (0.0119mol) Sodium was dissolved in 30mL of deionized water to make a solution of a certain concentration, and added dropwise to the flask, and after the addition was completed, stirred and reacted at 50°C for 5 hours. After the reaction was completed, the mixture was poured into water, and an orange precipitated solid (1) was precipitated. , filtered, washed and dried to obtain 1.64 g of 4,4'-thiobis(2-nitroaniline).
[0027] Step 2) Preparation of 3,3',4,4'-tetraaminodiphenylsulfide hydrochloride
[0028] 10.09g of stannous chloride dihydrate (SnCl 2 2H 2 O) join in the there-necked flask that 70mL concentrated hydrochloric acid is housed, stir, and the temperature in the flask is maintained on 40 ℃, wait fo...
Embodiment 2
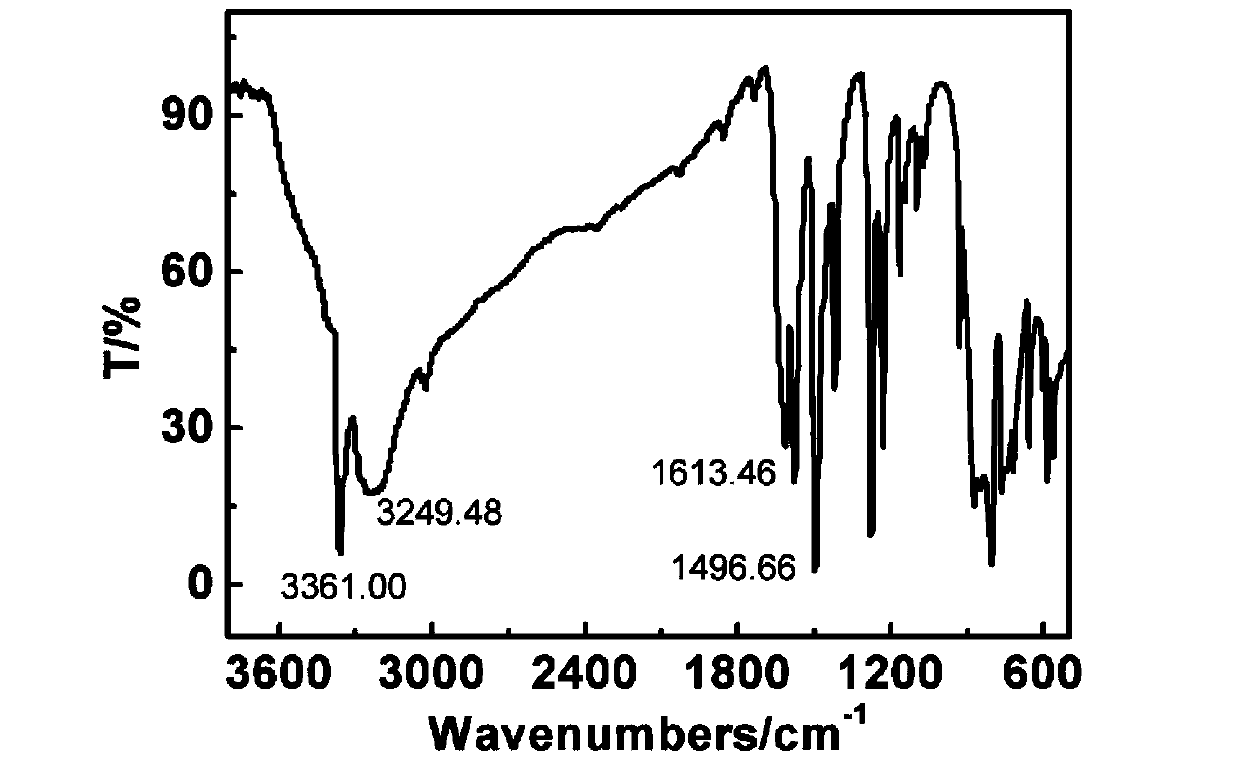
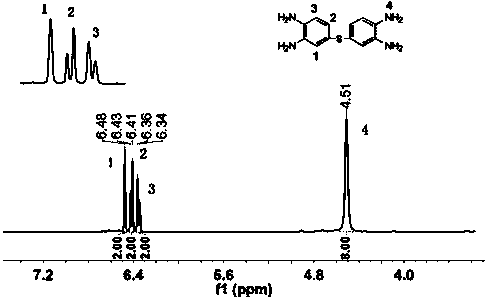
[0032] Preparation of 3,3',4,4'-tetraaminodiphenylsulfide
[0033] Add 40mL of N,N-dimethylformamide (DMF) and 2.85g (0.02mol) of 4-chloro-o-diphenylamine into a three-necked flask equipped with a thermometer, dropping funnel, and reflux condenser and stir evenly. Mix 9.70 g (0.024mol) of sodium sulfide was dissolved in 30mL of deionized water to form a solution of a certain concentration, and was added dropwise to the flask. After the addition was completed, it was stirred and reacted at 50-80°C for 6 hours. After the reaction, the mixture was poured into water. A light pink solid was precipitated, which was filtered, washed and purified to obtain 3,3',4,4'-tetraaminodiphenyl sulfide.
Embodiment 3
[0035] Step 1) Preparation of 4,4'-thiobis(o-dinitrobenzene)
[0036] Add 50 mL of N,N-dimethylformamide (DMF), 2.47 g (0.01 mol) of 5-bromo-2-nitroaniline into a three-necked flask equipped with a thermometer, dropping funnel, and reflux condenser, and stir well , Dissolve 4.85g (0.012mol) of sodium sulfide in 35mL of N,N-dimethylformamide (DMF) to make a solution with a certain concentration, add it dropwise to the flask, and stir at 80°C for reaction After 2 hours, the mixture was poured into water after the reaction, and an orange precipitated solid was precipitated. After filtering, washing and drying, 1.64 g of 4,4'-thiobis(o-dinitrobenzene) was obtained.
[0037] Step 2) Preparation of 3,3',4,4'-tetraaminodiphenylsulfide hydrochloride
[0038]Add 1.04g of 4,4'-thiobis(o-dinitrobenzene), 20mL of ethanol and 0.15g of Pd / C catalyst into a 50mL three-necked flask, blow nitrogen gas, stir in an ice bath, and add 5mL of Hydrazine hydrate. React at 40°C for 7 hours, let sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com