Application of preparation of pharmaceutical composition containing matrine and glycyrrhizic acid to treat psoriasis
A composition, the technology of glycyrrhizic acid, applied in the field of medicine, can solve the problems that patients are not suitable for use, and achieve the effect of remarkable effect and low toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Comparison of acute toxic effects of oxymatrine, glycyrrhizic acid and their compositions on mice
[0023] Kunming mice were randomly divided into a normal control group and a test drug group, with ten mice in each group, half male and half male. In addition to the normal control group, the test drug group was injected intraperitoneally (ip) with a large dose of oxymatrine, glycyrrhizin and the combination of the two components once, and the animals were observed continuously for 7 days, and the death time and number of animals were recorded.
[0024] The results showed that when the dose of oxymatrine was 950mg / kg, it had great toxicity, and 9 of the 10 mice died; when the dose of glycyrrhizin was 950mg / kg, 2 of the 10 mice died, while the oxidized Kushen minus: glycyrrhizin ratio 1:1 and combined dose of 950mg / kg and matrine: glycyrrhizin ratio of 2:1 and combined dosage of 950mg / kg, no animals died, oxidized matrine reduced: glycyrrhizin When the ratio of oxymatrine...
Embodiment 2
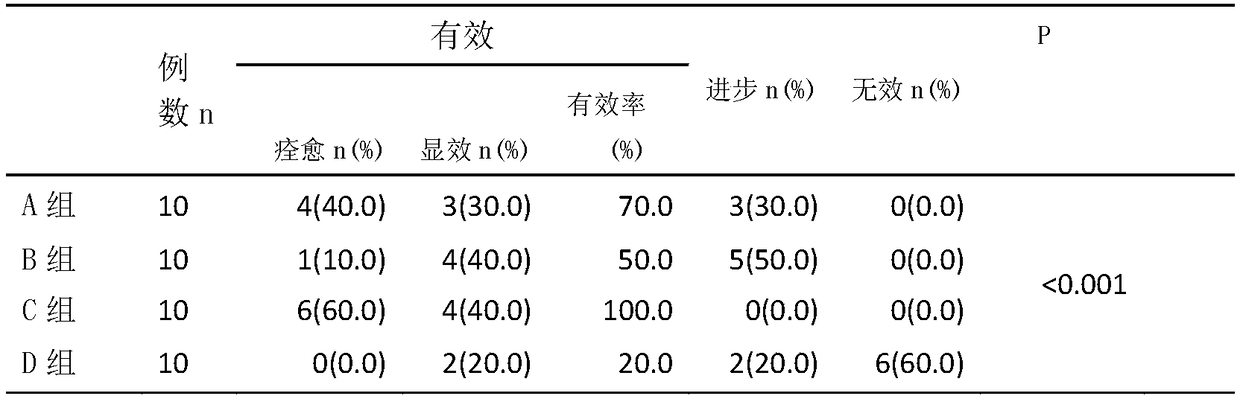
[0029] Observation on the curative effect of compound glycyrrhizin combined with oxymatrine in the treatment of psoriasis
[0030] 1 Materials and methods
[0031] 1.1 Clinical data 40 patients with psoriasis who were clinically diagnosed as psoriasis vulgaris, 20 males and 20 females, aged 24-58 years, had never received anti-psoriasis systemic treatment within one month, and had no photosensitivity disease , no serious visceral diseases such as liver and kidney dysfunction, no history of active tuberculosis, hypertension, diabetes, cataract, and pregnant and lactating women were excluded. The patients were randomly divided into four groups: oxymatrine treatment group (group A), compound glycyrrhizin treatment group (group B), oxymatrine combined with compound glycyrrhizin treatment group (group C), and control group (group D). group), ten cases in each group, half male and half male. There was no statistical difference among the four groups in terms of age, gender, and dis...
Embodiment 3
[0043] Oxymatrine 200mg
[0044] Diammonium Glycyrrhizinate 150mg
[0045] NaCl 0.9g
[0046] Appropriate amount of water for injection
[0047] 100ml each
[0048] Take NaCl, stir and dissolve with water for injection, then add oxymatrine and diammonium glycyrrhizinate respectively, dissolve completely while continuing to stir, add water for injection to the total amount, filter until clear, seal in a pot, and sterilize to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com