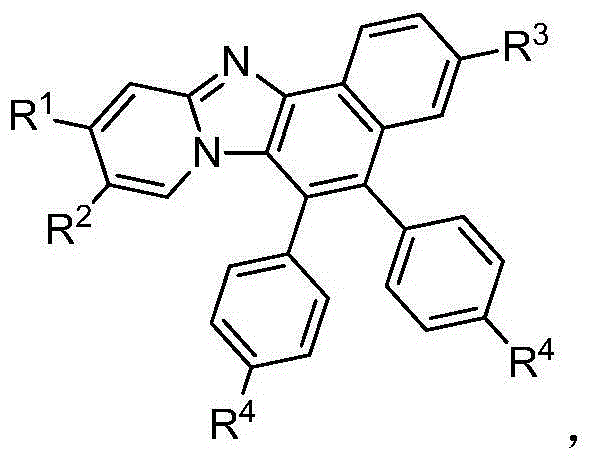
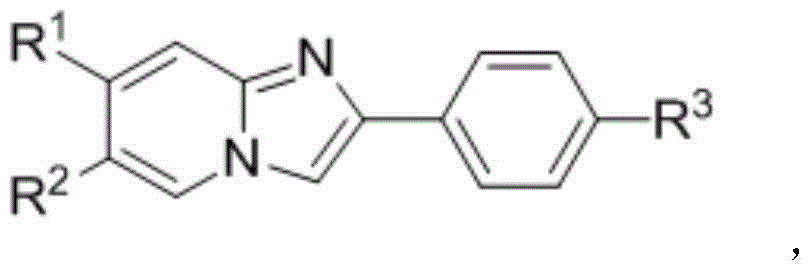
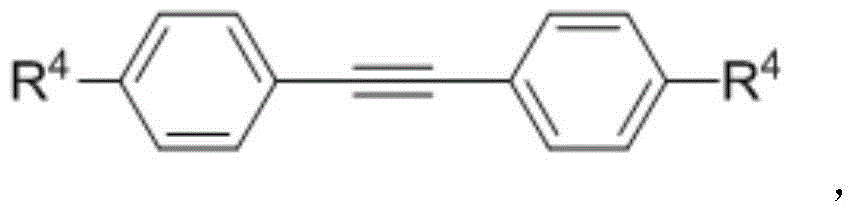
Naphthoimidazopyridine compound and preparation method thereof
A technology of naphthimidazoles and compounds, which is applied in the field of naphthimidazopyridine compounds and their preparation, can solve the problems of poor stability, low atom economy, expensive substrates, etc., and achieve the effect of easy purification and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of the compound diphenylnaphthoimidazopyridine:
[0030]
[0031] Add 0.1mmol of 2-phenylpyrido[1,2-a]imidazole, 0.2mmol of tolan, 0.005mmol of [{RhCl 2 Cp*} 2 ], and 0.12 mmol of Co(OAc) 2 4H 2 O, 1 mL of DMF (N,N-dimethylformamide). The reaction tube was sealed and reacted at 110° C. for 2 hours to obtain a black reaction solution. Concentrate the black reaction solution under reduced pressure, and separate by column chromatography (silica gel 200-300 mesh, eluent: ethyl acetate / petroleum ether=1 / 4) to obtain the target product diphenylnaphthoimidazopyridine. Pale yellow solid, yield 92%. 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 8.98 (dd, J = 0.4, 8.0Hz, 1H), 7.85 (dt, J = 0.8, 9.2Hz, 1H), 7.73-7.69 (m, 1H), 7.62 (d, J = 8.0 Hz,1H),7.53-7.48(m,1H),7.34-7.31(m,4H),7.29(brs,1H),7.28-7.27(m,2H),7.25-7.24(m,2H),7.23- 7.19(m,3H),6.50(dt,J=1.2,7.0Hz,1H). 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 148.0, 140.8, 138.6, 136.9, 133.6, 131.6, 131.4, 130.4, 128.5, 127.7...
Embodiment 2
[0033] Synthesis of compound bis(4-methylphenyl)naphthoimidazopyridine:
[0034]
[0035] Add 0.1mmol of 2-phenylpyrido[1,2-a]imidazole, 0.2mmol of 1,2-di(4-methylphenyl)acetylene, 0.005mmol of [{ RhCl 2 Cp*} 2 ], and 0.12 mmol of Co(OAc) 2 4H 2 O, 1 mL of DMF (N,N-dimethylformamide). The reaction tube was sealed and reacted at 110° C. for 2 hours to obtain a black reaction solution. Concentrate the black reaction solution under reduced pressure, and separate by column chromatography (silica gel 200-300 mesh, eluent: ethyl acetate / petroleum ether = 1 / 4) to obtain the target product bis(4-methylphenyl)naphthimidazo pyridine. Pale yellow solid, yield 80%. 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 8.97 (d, J = 8.0Hz, 1H), 7.84 (d, J = 9.2Hz, 1H), 7.69 (t, J = 7.2Hz, 1H), 7.62 (d, J = 8.4Hz ,1H),7.49(t,J=7.6Hz,1H),7.35-7.28(m,2H),7.14(s,4H),7.08(s,2H),7.07(s,2H),6.50(t, J=6.4Hz,1H),2.38(s,3H),2.33(s,3H). 13 C NMR (100MHz, CDCl 3): δ (ppm) 148.0, 140.8, 137.2, 136.0, 135.6, ...
Embodiment 3
[0037] Synthesis of compound bis(4-methoxyphenyl)naphthoimidazopyridine:
[0038]
[0039] Add 0.1mmol of 2-phenylpyrido[1,2-a]imidazole, 0.2mmol of 1,2-di(4-methoxyphenyl)acetylene, 0.005mmol of [ {RhCl 2 Cp*} 2 ], and 0.12 mmol of Co(OAc) 2 4H 2 O, 1 mL of DMF (N,N-dimethylformamide). The reaction tube was sealed and reacted at 110° C. for 2 hours to obtain a black reaction solution. Concentrate the black reaction solution under reduced pressure, and separate by column chromatography (silica gel 200-300 mesh, eluent: ethyl acetate / petroleum ether=1 / 4) to obtain the target product bis(4-methoxyphenyl)naphthimidazole And pyridine. Pale yellow solid, 84% yield. 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 8.96 (dd, J = 0.4, 8.0Hz, 1H), 7.84 (dt, J = 0.8, 9.2Hz, 1H), 7.72-7.68 (m, 1H), 7.64 (d, J = 8.4 Hz,1H),7.52-7.48(m,1H),7.38(d,J=7.2Hz,1H),7.31(ddd,J=1.2,6.8,9.2Hz,1H),7.15(d,J=8.8Hz ,2H),7.10(d,J=8.8Hz,2H),6.88(d,J=8.8Hz,2H),6.81(d,J=8.8Hz,2H),6.53(dt,J=1.2,6.8Hz ,1H),3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com