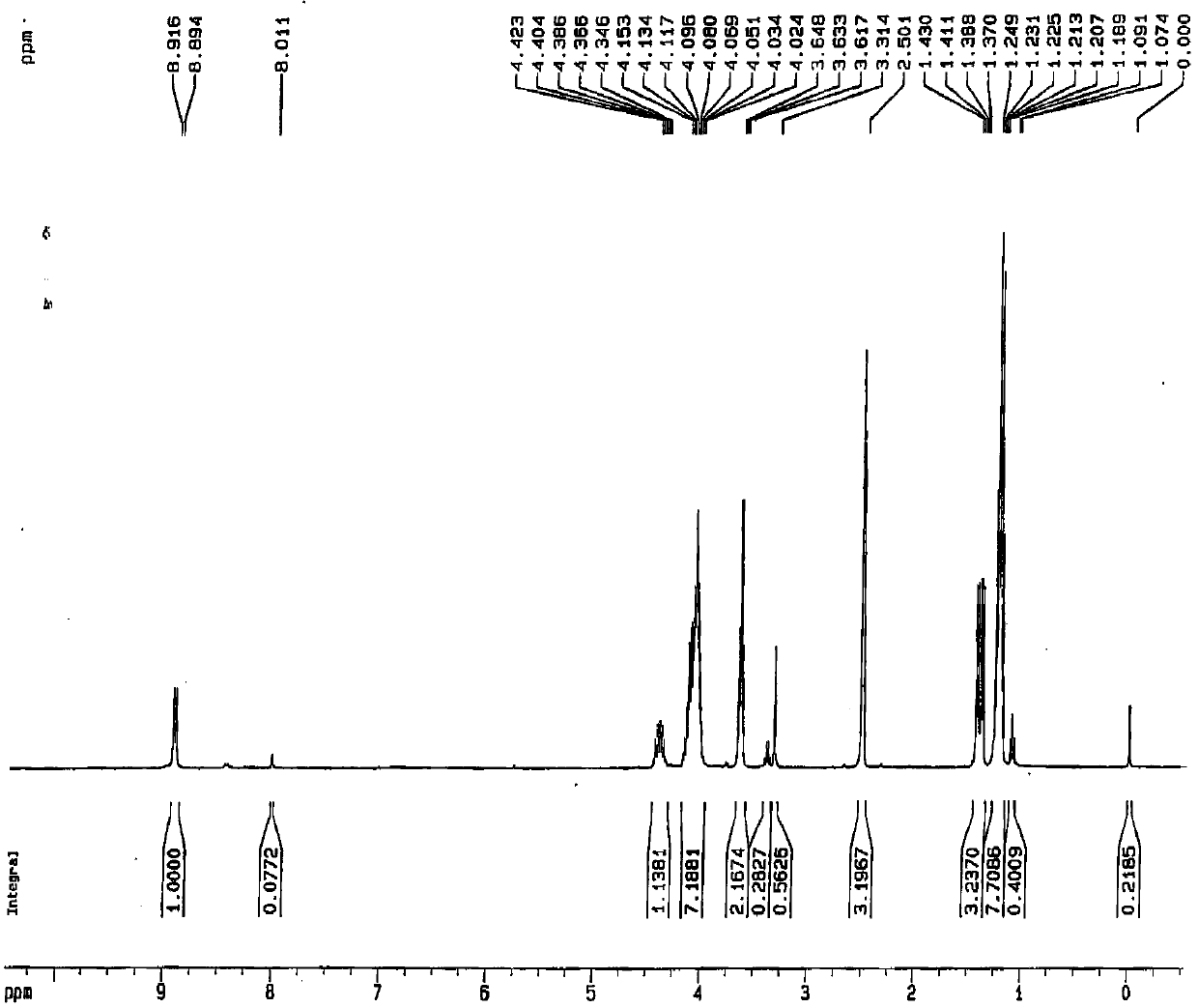
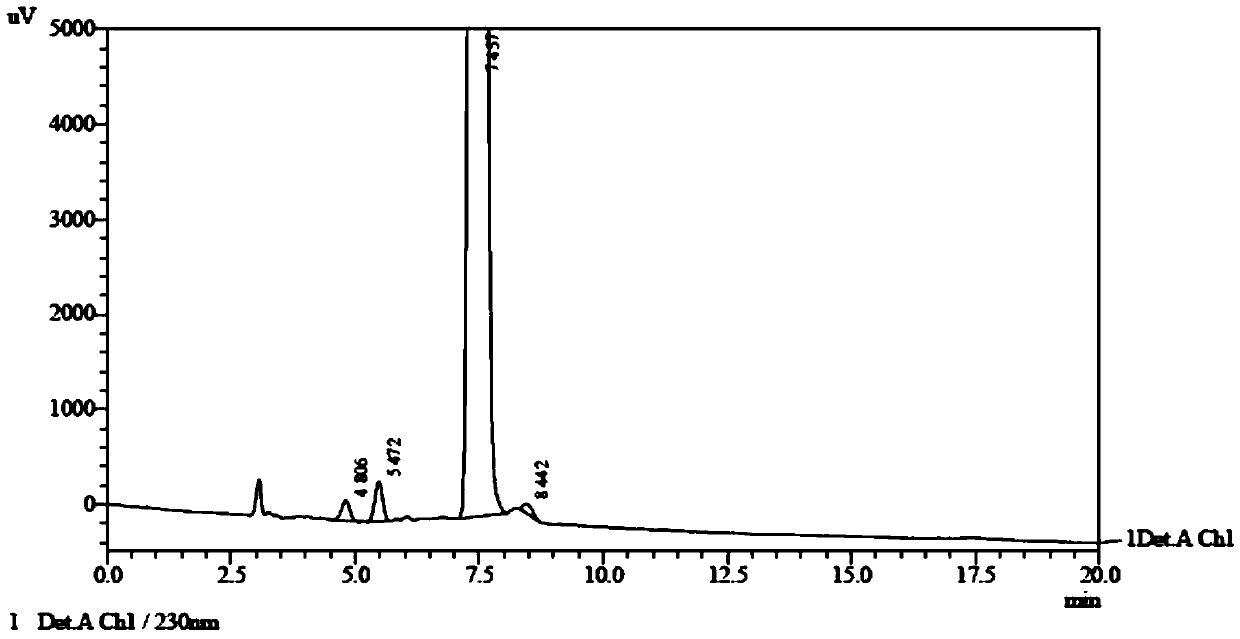
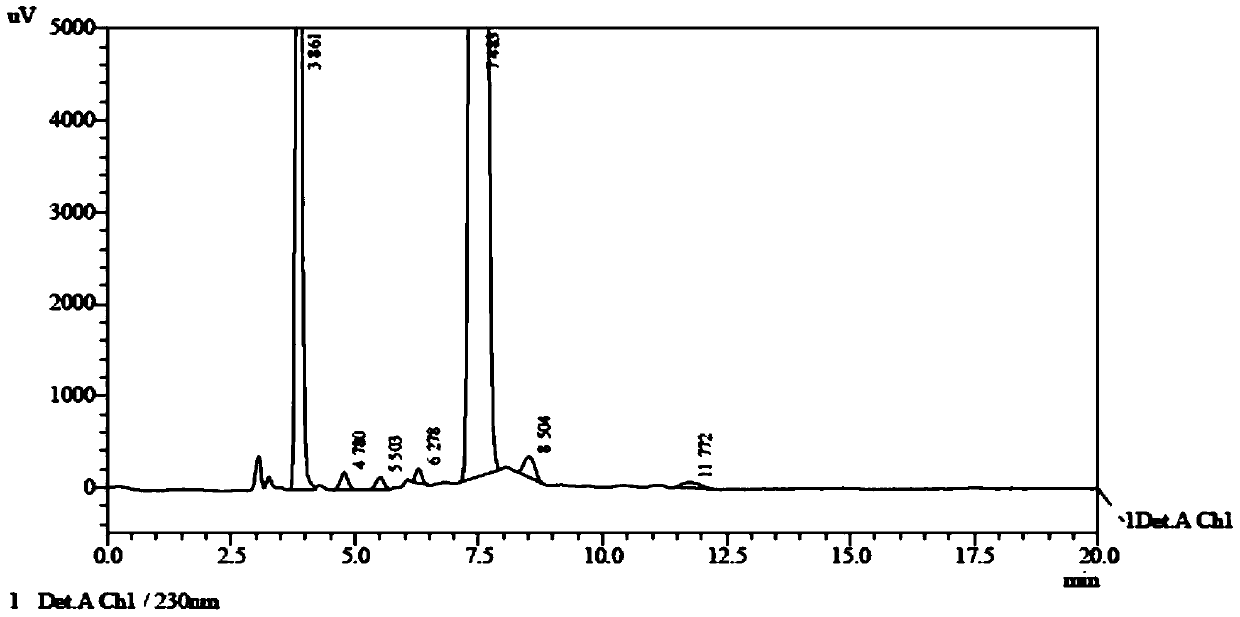
A kind of preparation method of falmustine
A technology of formustine and reaction medium, which is applied in the field of medicinal chemistry, can solve the problems of low product purity and low yield, and achieve the effect of high purity and high yield, which is suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a kind of preparation method of formustine, comprises the following steps:
[0028] a) Aminolysis reaction of 1-aminoethyl diethyl phosphate and carmustine in the reaction medium to obtain the compound shown in formula (II); the reaction medium includes water;
[0029] b) Nitrosating the compound represented by formula (II) into formustine;
[0030]
[0031] The present invention uses 1-aminoethyl phosphate diethyl ester and carmustine as raw materials, wherein the structural formula of 1-aminoethyl phosphate diethyl ester is:
[0032]
[0033] The chemical name of carmustine is: 1,3-bis(2-chloroethyl)-1-nitrosourea, and the structural formula is:
[0034]
[0035] The 1-aminoethyl phosphate diethyl ester and carmustine described in the present invention are raw materials well known to those skilled in the art, and the present invention has no particular limitation thereto. The molar ratio of the 1-aminoethyl diethyl phosphate to the ca...
Embodiment 1
[0045] (1) Put 5.10 g (0.0279 mol) of 1-aminoethyl phosphate diethyl ester in a 50 mL reaction flask, add 4.68 mL of purified water, then add 2.0 g (0.0093 mol) of carmustine at room temperature, and React for 2 hours, then cool to room temperature, wash with 10% dilute hydrochloric acid, extract with dichloromethane, dry, evaporate to dryness under reduced pressure, then wash with 20 mL of purified water, extract with dichloromethane (3×20 mL), combine the extracts, Dry over sodium sulfate, filter, and evaporate to dryness under reduced pressure to obtain the reaction product.
[0046](2) Add 8.0mL of formic acid to the reaction product obtained in the above steps, keep the reaction temperature at 0°C~5°C, add 1.4g (3mol) of sodium nitrite in batches, it takes 1.5h to complete the addition, and carry out nitrosation for 0.5h , then distill off formic acid under reduced pressure at less than 35°C, dissolve the residue in 20mL of dichloromethane, wash with purified water (20mL×...
Embodiment 2
[0049] (1) Put 3.98g (0.0186mol) of 1-aminoethyl diethylphosphate in a 50mL reaction flask, add 4.68mL of purified water, then add 2.0g (0.0093mol) of carmustine at room temperature, and React for 2 hours, then cool to room temperature, wash with 10% dilute hydrochloric acid, extract with dichloromethane, dry, evaporate to dryness under reduced pressure, then wash with 20 mL of purified water, extract with dichloromethane (3×20 mL), combine the extracts, Dry over sodium sulfate, filter, and evaporate to dryness under reduced pressure to obtain the reaction product.
[0050] (2) Add 8.0mL of formic acid to the reaction product obtained in the above steps, keep the reaction temperature at 0°C~5°C, add 1.4g (3mol) of sodium nitrite in batches, it takes 1.5h to complete the addition, and carry out nitrosation for 0.5h , then distill off formic acid under reduced pressure at less than 35°C, dissolve the residue in 20mL of dichloromethane, wash with purified water (20mL×3), dry over...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com