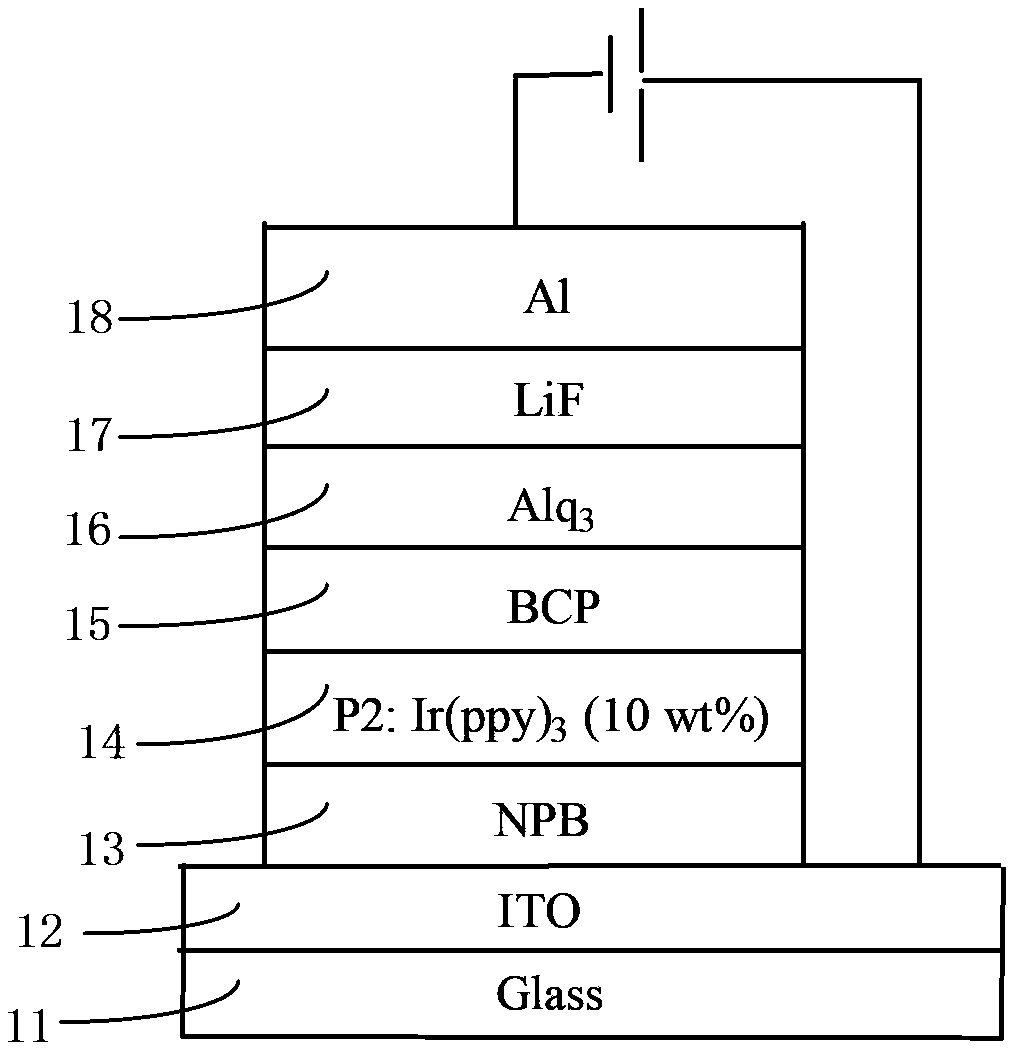
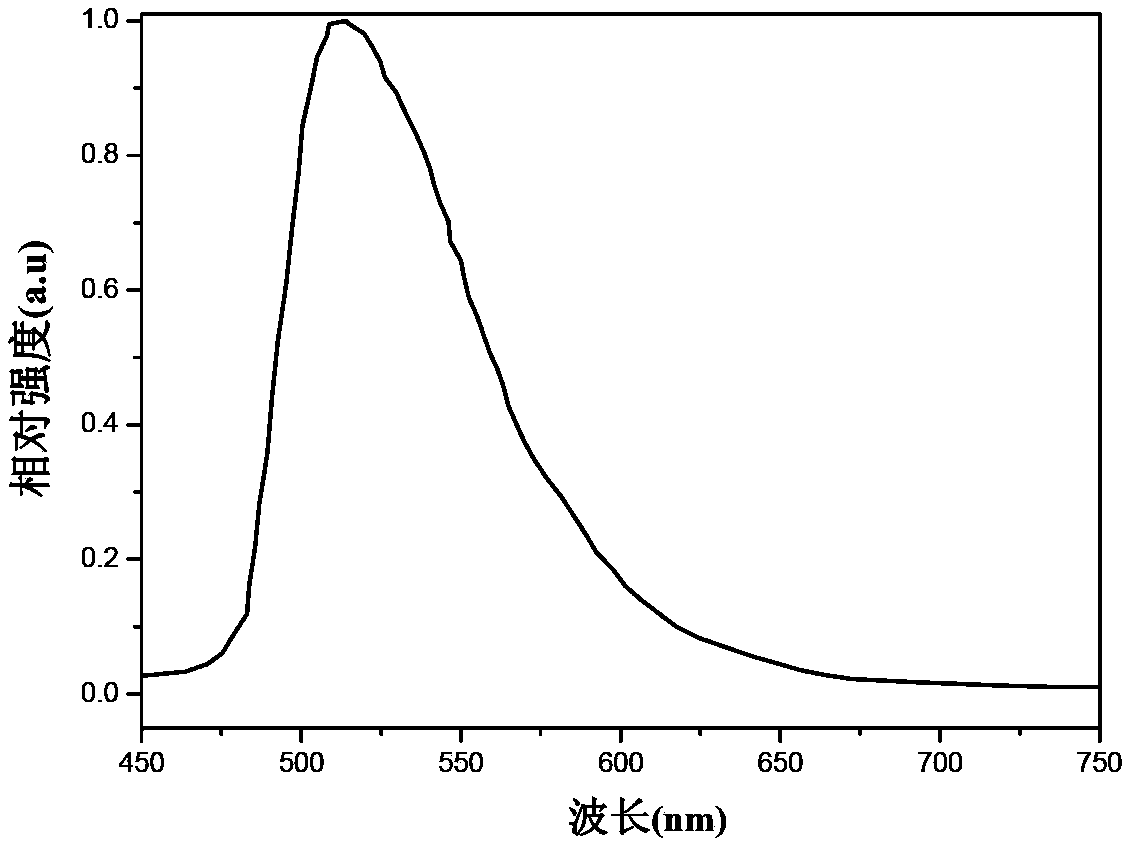
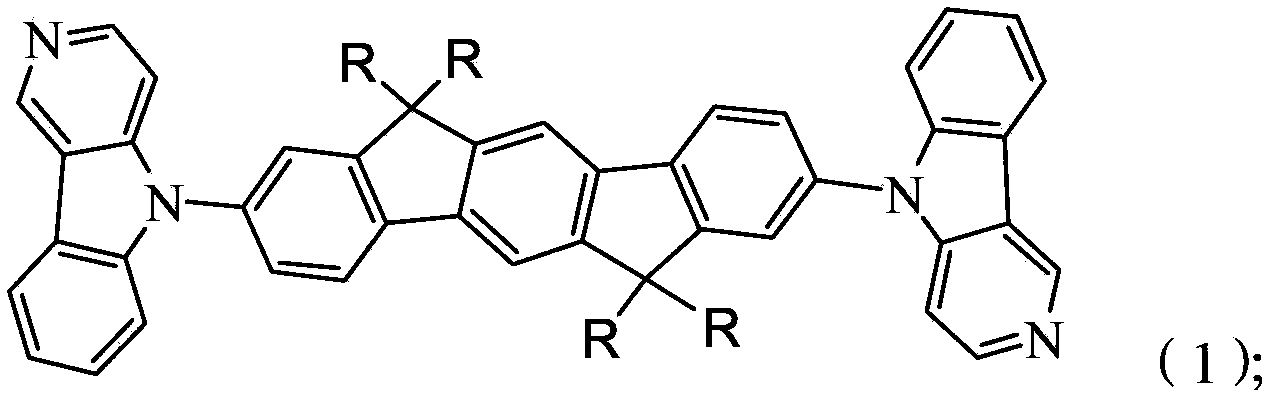
Organic electrophosphorescent main body material as well as preparation method thereof and organic electroluminescence device
A technology of electrophosphorescence and host materials, applied in the direction of electric solid devices, luminescent materials, semiconductor devices, etc., can solve the problems of large gaps in electron transport performance, low glass transition temperature, and affecting device life, etc., to achieve easy preparation, Good film-forming performance and prolonging device life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This example provides a 2,8-bis(5H-pyrido[4,3-b]indolyl-5-yl)-6,12-dihydroindeno[1,2-B]fluorene (PIIF), whose chemical structure is shown in formula (2):
[0049]
[0050] The preparation steps of above-mentioned PIIF are as follows:
[0051] S10, providing compound A (5H-pyrido[4,3-b]indole) represented by the following structural formula and compound B1 (2,8-dibromo-6,12-dihydroindeno[1,2-B] fluorene):
[0052]
[0053] S20, preparation of PIIF by coupling reaction
[0054] Under nitrogen protection, add 6mmol of A, 3mmol of B1, 25mL of toluene, and add 0.09mmol of Pd(OAc) under stirring 2 , 5.4 mmol of t-BuONa, 0.27 mmol of (t-Bu) 3 PHBF 4 , reflux and stir at 110°C, perform coupling reaction for 48 hours, and cool to obtain a reaction solution containing PIIF product;
[0055] Separation and purification of S30 and PIIF
[0056] The cooled reaction solution in S20 was mixed with a saturated ammonium chloride aqueous solution, and the mixed solution was ...
Embodiment 2
[0060] This example provides a 2,8-bis(5H-pyrido[4,3-b]indolyl-5-yl)-6,6,12,12-tetramethyl-6,12- Dihydroindeno[1,2-B]fluorene (PIMIF), its chemical structure is shown in formula (3):
[0061]
[0062] The preparation steps of above-mentioned PIMIF are as follows:
[0063] S10, provide compound A (5H-pyrido[4,3-b]indole) represented by the following structural formula and compound B2 (2,8-dibromo-6,6,12,12-tetramethyl-6,12 -dihydroindeno[1,2-B]fluorene):
[0064]
[0065] S20, preparation of PIMIF by coupling reaction
[0066] Under the protection of nitrogen, add 7mmol of A, 3mmol of B2, 25mL of xylene in sequence, add 0.03mmol of CuI, 0.09mmol of phenanthroline, and 18.0mmol of KOH under stirring, reflux and stir at 125-130°C, and the coupling reaction After cooling for 24 hours, a reaction liquid containing PIMIF was obtained.
[0067] Separation and purification of S30 and PIMIF
[0068] Mix the cooled reaction solution in S20 with dilute hydrochloric acid soluti...
Embodiment 3
[0072] This example provides a 2,8-bis(5H-pyrido[4,3-b]indolyl-5-yl)-6,6,12,12-tetra-n-propyl-6,12 -Dihydroindeno[1,2-B]fluorene (PIPIF), the chemical structure of which is shown in formula (4):
[0073]
[0074] The preparation of the above PIPIF is as follows:
[0075] S10, providing compound A (5H-pyrido[4,3-b]indole) represented by the following structural formula and compound B3 (2,8-dibromo-6,6,12,12-tetra-n-propyl-6, 12-dihydroindeno[1,2-B]fluorene):
[0076]
[0077] S20, preparation of PIPIF by coupling reaction
[0078] Add 9mmol of A, 3mmol of B3, 25mL of xylene sequentially under the protection of argon, add 9mmol of Cu powder, 1.0mmol of 18-crown-6, 27.0mmol of K under stirring 2 CO 3 , 15mL o-dichlorobenzene, reflux and stir at 185° C., and perform a coupling reaction for 72 hours to obtain a reaction solution containing PIPIF.
[0079] Separation and purification of S30 and PIPIF
[0080] First use a short silica gel column to heat filter the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com