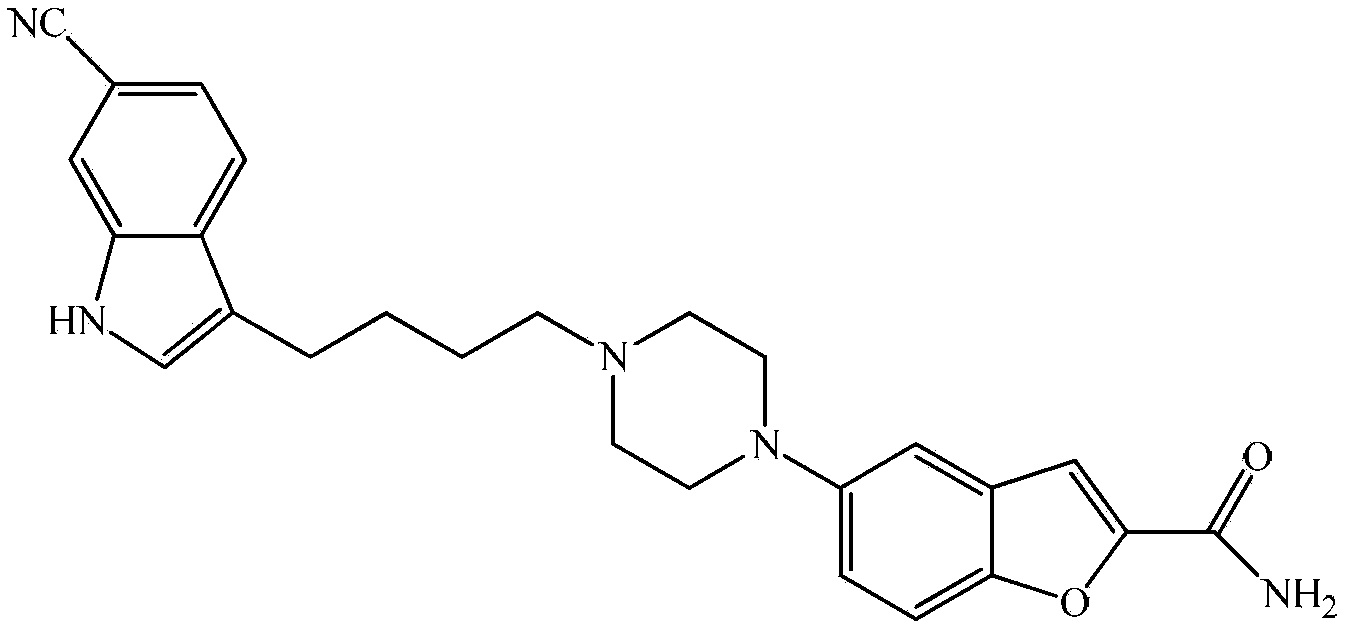
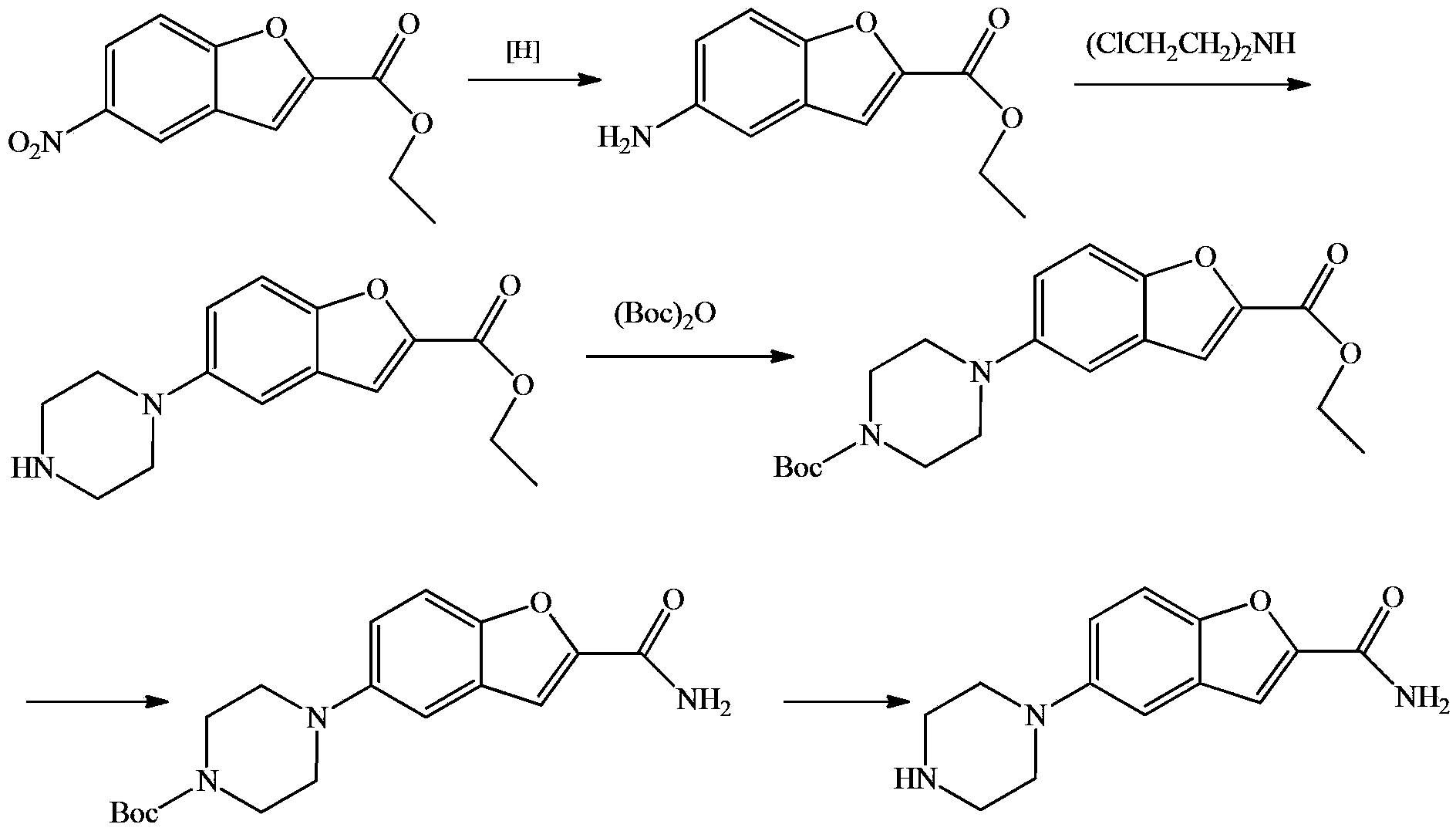
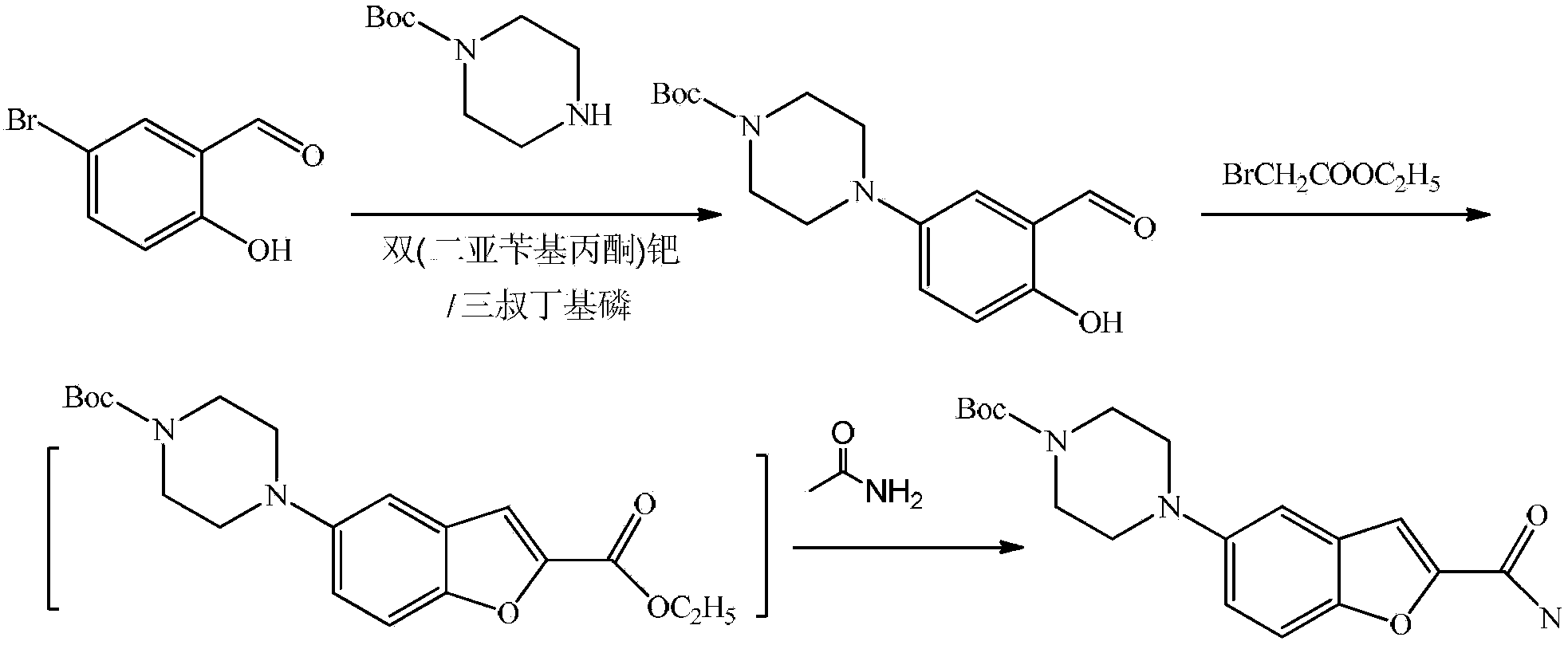
Substituted piperazine compound and preparation method for intermediate of vilazodone
A compound and piperazine technology, applied in the field of medicine, can solve the problems of environmental hazards, high preparation cost, long reaction route, etc., and achieve the effects of environmental protection route, simple operation and outstanding yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 5-(4-acetylpiperazin-1-yl)-2-hydroxybenzaldehyde
[0037] Add 269g (2.8mol) of methanesulfonic acid, 88g (0.4mol) of 4-(4-acetylpiperazin-1-yl)phenol and 56g (0.4mol) of urotropine into a 2L four-necked flask, stir and heat, The temperature was raised to 110° C., and the reaction was carried out for 3 hours. After the reaction is complete, keep stirring, add 1L of water, add 1L of ethyl acetate after the addition, then pour into a separation funnel, separate the ethyl ester layer, wash with water, add anhydrous magnesium sulfate to dry, filter, recover the ethyl acetate under reduced pressure, evaporate Dry to get 50g of oil. The yield is 50%. NMR(DMSO)δ2.0(S,3H), 3.30-3.60(d,4H), 3.81(s,4H), 6.87-7.13(d,1H), 7.65-7.76(d,2H), 10.26(s , 1H, -CHO)
[0038] The resulting solid can be isolated by dissolving in ethanol, adding hydrochloric acid ethanol solution dropwise, and drying the corresponding hydrochloride sample.
Embodiment 2
[0040] Preparation of 5-(4-tert-butoxycarbonylpiperazin-1-yl)-2-hydroxybenzaldehyde
[0041] Add 947g (2.8mol) of polyphosphoric acid, 78g (0.28mol) of 4-(4-tert-butoxycarbonylpiperazin-1-yl)phenol and 78g (0.56mol) of urotropine into a 2L four-neck flask , stirred and heated, the temperature was raised to 90°C, and the reaction was carried out for 1 hour. After the reaction is complete, keep stirring, add 1L of water, add 1L of ethyl acetate after the addition, then pour into a separation funnel, separate the ethyl ester layer, wash with water, add anhydrous magnesium sulfate to dry, filter, recover the ethyl acetate under reduced pressure, evaporate Dry to get 38g of oil. The yield is 45%. mp84-86°C; MS306 (M+), 250 (100%), 233, 176, 164.
Embodiment 3
[0043] Preparation of 5-(4-benzylpiperazin-1-yl)-2-hydroxybenzaldehyde
[0044] Add 319g (2.8mol) of trifluoroacetic acid, 150g (0.56mol) of 4-(4-benzylpiperazin-1-yl)phenol and 78g (0.56mol) of urotropine into a 2L four-neck flask, stir and heat , heated up to 100°C, and reacted for 2 hours. After the reaction is complete, keep stirring, add 1L of water, add 1L of ethyl acetate after the addition, then pour into a separation funnel, separate the ethyl ester layer, wash with water, add anhydrous magnesium sulfate to dry, filter, recover the ethyl acetate under reduced pressure, evaporate 69g of oil was obtained after drying. The yield is 42%. mp 101-103°C; MS 296 (M+), 205, 119, 91 (100%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com