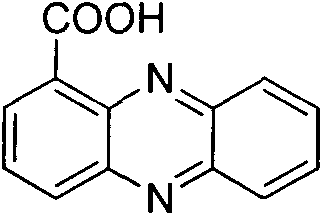
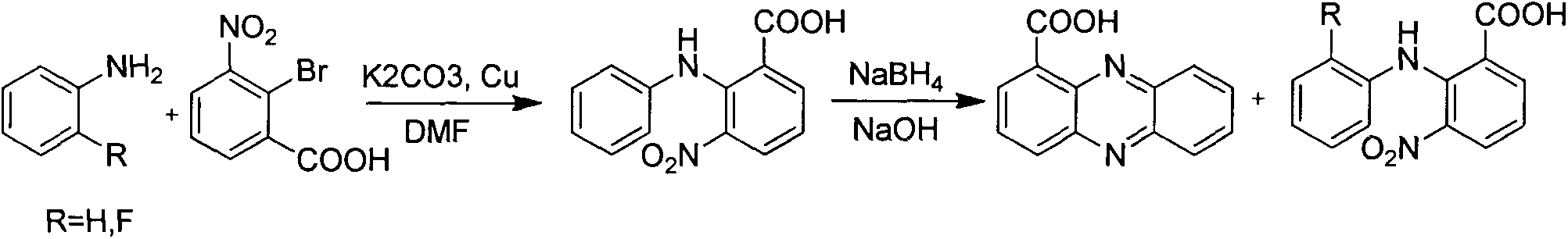
Method for synthesizing phenazine-1-carboxylic acid
A technology of carboxylic acid and methylphenazine, applied in the field of chemistry, can solve the problems of cumbersome processing, unsuitable for large-scale production, and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of 1-bromomethylphenazine (compound III)
[0027] Add (1.95g, 10.0mmol) compound IV in a 100mL four-necked flask, 20.0ml of carbon tetrachloride, N-bromosuccinimide (0.90g, 5mmol), benzoyl peroxide (0.39g, 1.6 mmol). The mixture was then heated to reflux, at which temperature N-bromosuccinimide (0.81 g, 5 mmol) was added portionwise. After the addition was complete, the reaction was carried out for 5 hours (TLC followed the reaction). The reaction mixture was poured into water (50.0 mL), and the obtained mixture was extracted with ethyl acetate (40.0 mL×3). After drying over anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure to obtain a yellow solid, which was finally subjected to silica gel column chromatography (petroleum Ether:ethyl acetate=30:1) isolated to obtain 1.02 g of compound III with a melting point of 163-165° C. and a yield of 40.0%.
Embodiment 2
[0028] Embodiment 2: the synthesis of 1-bromomethylphenazine (compound III)
[0029] Add (1.95g, 10.0mmol) compound IV in a 100mL four-necked flask, carbon tetrachloride 20.0ml, N-bromosuccinimide (1.21g, 6.5mmol), benzoyl peroxide (0.39g , 1.6 mmol). The mixture was then heated to reflux at which temperature N-bromosuccinimide (1.32 g, 6.5 mmol) was added portionwise. After the addition was complete, the reaction was carried out for 4 hours (TLC followed the reaction). The reaction mixture was poured into water (50.0 mL), and the obtained mixture was extracted with ethyl acetate (40.0 mL×3). After drying over anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure to obtain a yellow solid, which was finally subjected to silica gel column chromatography (petroleum Ether: ethyl acetate = 30:1) isolated to obtain 2.09 g of compound III with a melting point of 163-165° C. and a yield of 76.1%.
Embodiment 3
[0030] Embodiment 3: the synthesis of 1-bromomethylphenazine (compound III)
[0031] Add (1.95g, 10.0mmol) compound IV in a 100mL four-necked flask, carbon tetrachloride 20.0mL, N-bromosuccinimide (1.35g, 7.5mmol), benzoyl peroxide (0.39g , 1.6 mmol). The mixture was then heated to reflux at which temperature N-bromosuccinimide (1.35 g, 7.5 mmol) was added portionwise. After the addition was complete, the reaction was carried out for 3.5 hours (TLC followed the reaction). The reaction mixture was poured into water (50.0 mL), and the obtained mixture was extracted with ethyl acetate (40.0 mL×3). After drying over anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure to obtain a yellow solid, which was finally subjected to silica gel column chromatography (petroleum Ether:ethyl acetate=30:1) isolated to obtain 2.48g of compound III with a melting point of 163-165°C and a yield of 91.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com