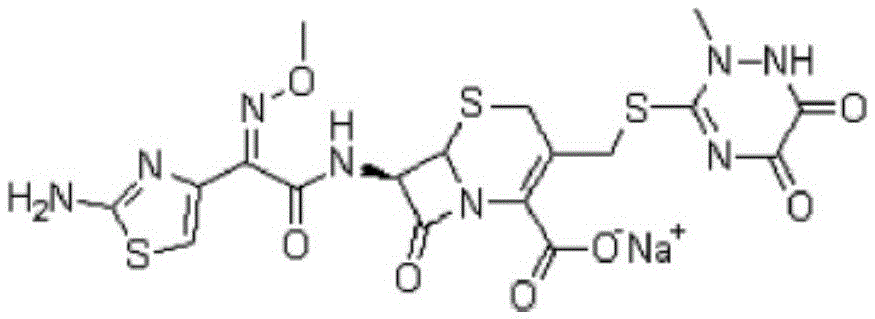
A kind of refining method of ceftriaxone sodium crude product
A technology of ceftriaxone sodium and a refining method is applied in the refining field of preparing fine ceftriaxone sodium and crude crystalline ceftriaxone sodium, and can solve the problems affecting the quality of ceftriaxone sodium, high product color grade, and ineffectiveness of ceftriaxone sodium. Stability and other issues, to achieve the effect of low price, simple operation and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
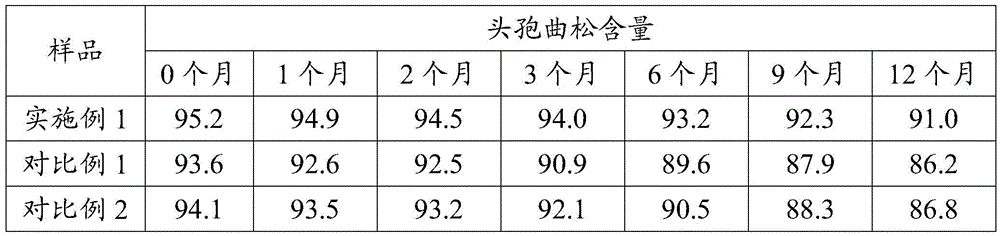
Embodiment 1
[0026] The refining method of ceftriaxone sodium, the steps are as follows:
[0027] (1) Weigh 50g of crude ceftriaxone sodium and 0.5g of 3,4-difluorobenzonitrile, add 100ml of dichloromethane, 40ml of ethanol and 10ml of water for injection, stir for 30min at room temperature, add 1g of activated carbon for decolorization 20min.
[0028] (2) The above solution is filtered through nitrogen pressure, and activated carbon and other impurities are removed by a microporous membrane. Then use the same proportion of mixed solvents to wash the suction filter flask, wash the filter cake, and transfer them to the crystallizer together.
[0029] (3) When the solution in the crystallizer reaches the crystallization temperature, start to drop fresh acetone, and after the acetone has been added dropwise, add sterile grade ceftriaxone sodium crystal seeds, and crystallize for 4 hours.
[0030] (4) Vacuum suction filtration is performed after dissolution and crystallization, the filter ca...
Embodiment 2
[0042] The refining method of ceftriaxone sodium, the steps are as follows:
[0043] (1) Weigh 50g of crude ceftriaxone sodium and 0.5g of 3,4-difluorobenzonitrile, add 100ml of dichloromethane, 50ml of ethanol and 10ml of water for injection, stir for 30min at room temperature, add 1g of activated carbon for decolorization 20min.
[0044] (2) The above solution is filtered through nitrogen pressure, and activated carbon and other impurities are removed by a microporous membrane. Then use the same proportion of mixed solvents to wash the suction filter flask, wash the filter cake, and transfer them to the crystallizer together.
[0045] (3) When the solution in the crystallizer reached the crystallization temperature, start to drop fresh acetone, after the acetone was added dropwise, add sterile grade ceftriaxone sodium crystal seeds, and crystallize for 5 hours.
[0046] (4) Vacuum suction filtration is performed after dissolution and crystallization, the filter cake is was...
Embodiment 3
[0048] The refining method of ceftriaxone sodium, the steps are as follows:
[0049] (1) Weigh 50g of crude ceftriaxone sodium and 0.5g of 3,4-difluorobenzonitrile, add 100ml of dichloromethane, 40ml of ethanol and 20ml of water for injection, stir for 30min at room temperature, add 1g of activated carbon for decolorization 20min.
[0050](2) The above solution is filtered through nitrogen pressure, and activated carbon and other impurities are removed by a microporous membrane. Then use the same proportion of mixed solvents to wash the suction filter flask, wash the filter cake, and transfer them to the crystallizer together.
[0051] (3) When the solution in the crystallizer reached the crystallization temperature, start to drop fresh acetone, after the acetone was added dropwise, add sterile grade ceftriaxone sodium crystal seeds, and crystallize for 5 hours.
[0052] (4) Vacuum suction filtration is performed after dissolution and crystallization, the filter cake is wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com