A kind of β-chloroethylnitrosourea compound and its synthesis method and application
A technology of chloroethyl nitroso compounds, which is applied in the fields of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc. It can solve the problems of undiscovered compounds, non-targeted inhibitory effects, and increased toxic and side effects of chemotherapy drugs. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
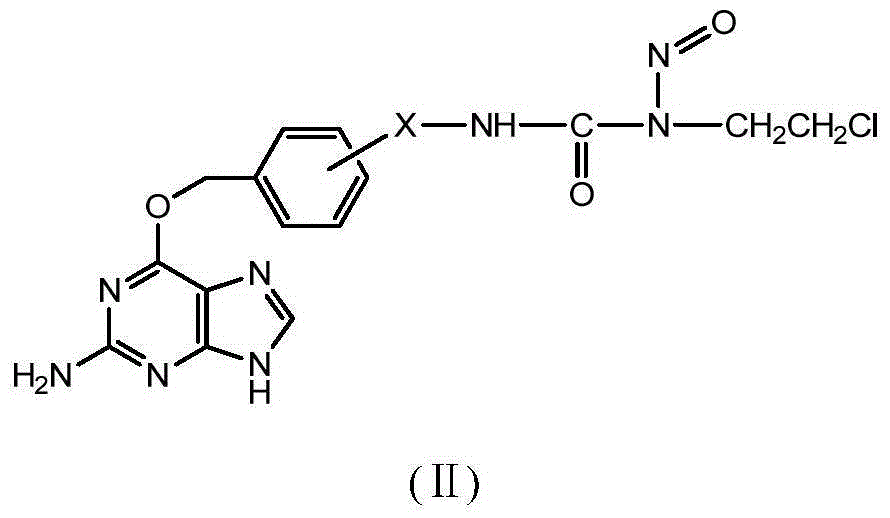
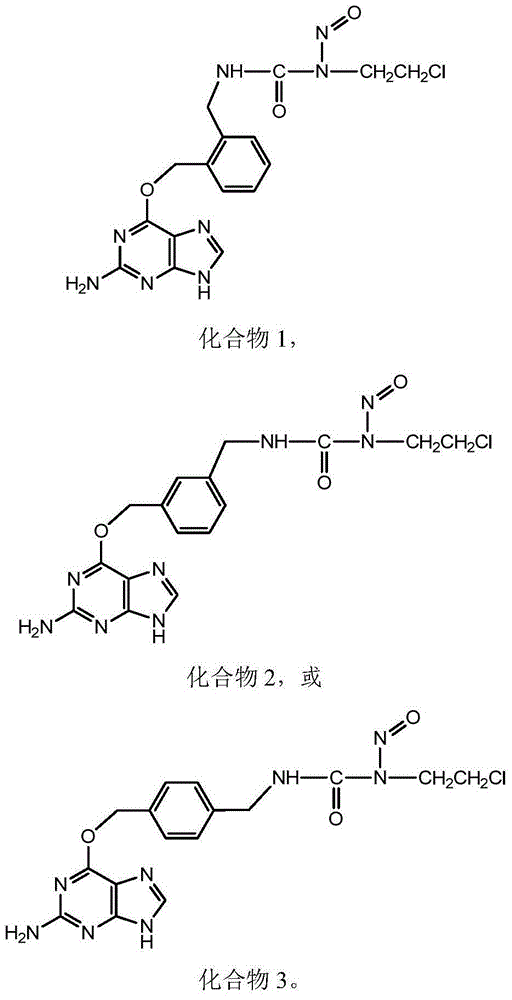
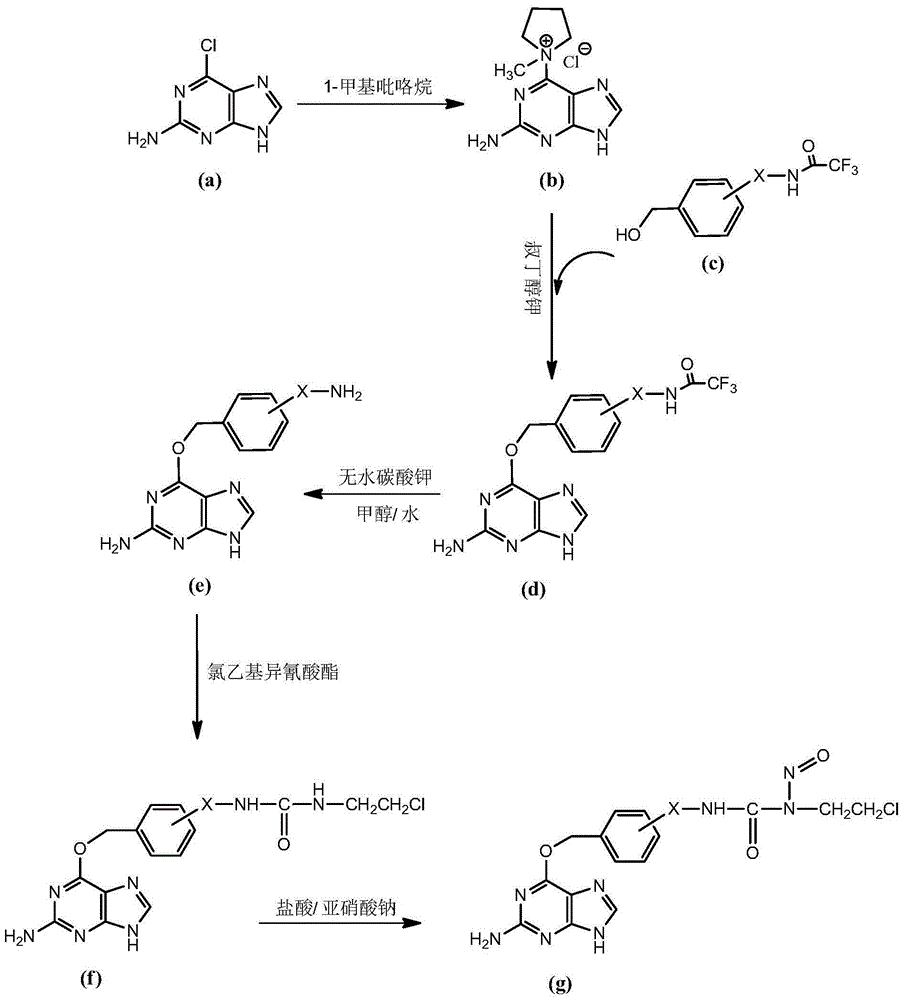
[0086] Embodiment 1:1-(O 6 -Synthesis of [2-(aminomethyl)benzyl]guanine)-3-(2-chloroethyl)-1-nitrosourea (Compound 1).
[0087] (1) Synthesis of 1-(2-amino-9-hydrogen-purine-6-)-1-methylpyrrolidine chloride
[0088] Dissolve 2g of 2-amino-6-chloropurine (11.8mmol) in 40mL of N,N-dimethylformamide, add 2.8mL of 1-methylpyrrolidine (26.4mmol), and mix and stir for 18 hours at 25°C. After the reaction is complete, Add 4mL of acetone, precipitation occurs, filter, the collected solid is washed twice with ether, and vacuum-dried to obtain 1-(2-amino-9-hydrogen-purine-6-)-1-methylpyrrolidine chloride ( 2.6 g, 8.2 mmol), yield 66%.
[0089] UV lambda: 289nm;
[0090] IR (KBr tablet) v / cm -1 : 3445.4(NH), 3274.9(NH 2 ), 2974.9(C-H), 1719.3(C=N), 1552.7(C=C);
[0091] 1 H-NMR (DMSO-d 6 )δ: 2.04(m,2H,CH 2 ),2.23(m,2H,CH 2 ),3.64(s,3H,CH 3 ),3.94(m,2H,CH 2 N + ),4.58(m,2H,CH 2 N + ),7.13(s,2H,NH 2 ),8.31(s,1H,H8),12.99(s,1H,H9);
[0092] ESI-MS: 255[M+H] + .
[0093] (...
Embodiment 2
[0126] Embodiment 2:1-(O 6-Synthesis of [3-(aminomethyl)benzyl]guanine)-3-(2-chloroethyl)-1-nitrosourea (Compound 2).
[0127] (1) Synthesis of 1-(2-amino-9-hydrogen-purine-6-)-1-methylpyrrolidine chloride: the method is the same as step (1) in Example 1.
[0128] (2) 3-[(trifluoroacetamido)methyl]-benzyl alcohol
[0129] Dissolve 3.4g of 3-(aminomethyl)-benzyl alcohol (24.8mmol) and 3.52mL of triethylamine (24.8mmol) in 50mL of anhydrous methanol, and add 3.92mL of ethyl trifluoroacetate (32.8mL) dropwise under argon protection. mmol), stirred and reacted at 25° C. for 2 hours, after the reaction was finished, 50 mL of ethyl acetate and 50 mL of water were added for extraction, the ethyl acetate layer was washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent was removed in vacuo to obtain 3 -[(trifluoroacetamido)methyl]-benzyl alcohol crude product, the crude product was purified by silica gel column chromatography, the...
Embodiment 3
[0161] Example 3: 1-(O 6 Synthesis of -[4-(aminomethyl)benzyl]guanine)-3-(2-chloroethyl)-1-nitrosourea (compound 3)
[0162] (1) Synthesis of 1-(2-amino-9-hydrogen-purine-6-)-1-methylpyrrolidine chloride: the method is the same as step (1) in Example 1.
[0163] (2) 4-[(trifluoroacetamido)methyl]-benzyl alcohol
[0164] 2.55g of 4-(aminomethyl)-benzyl alcohol (18.6mmol) and 2.64mL of triethylamine (18.6mmol) were dissolved in 25mL of anhydrous methanol, and 2.94mL of ethyl trifluoroacetate (24.6 mmol), stirred at 25°C for 2 hours. After the reaction was completed, 35 mL of ethyl acetate and 35 mL of water were added for extraction, the ethyl acetate layer was washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent was removed in vacuo to obtain 4-[(trifluoroacetamido)formazan base]-benzyl alcohol crude product. The crude product was purified by silica gel column chromatography, and the eluent was ethyl acetate / cyclohexan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com