Agent for reducing adverse side effects of kinase inhibitor
A technology of kinase inhibitors and side effects, applied in the field of anticancer drugs, can solve the problems of side effects and serious anticancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
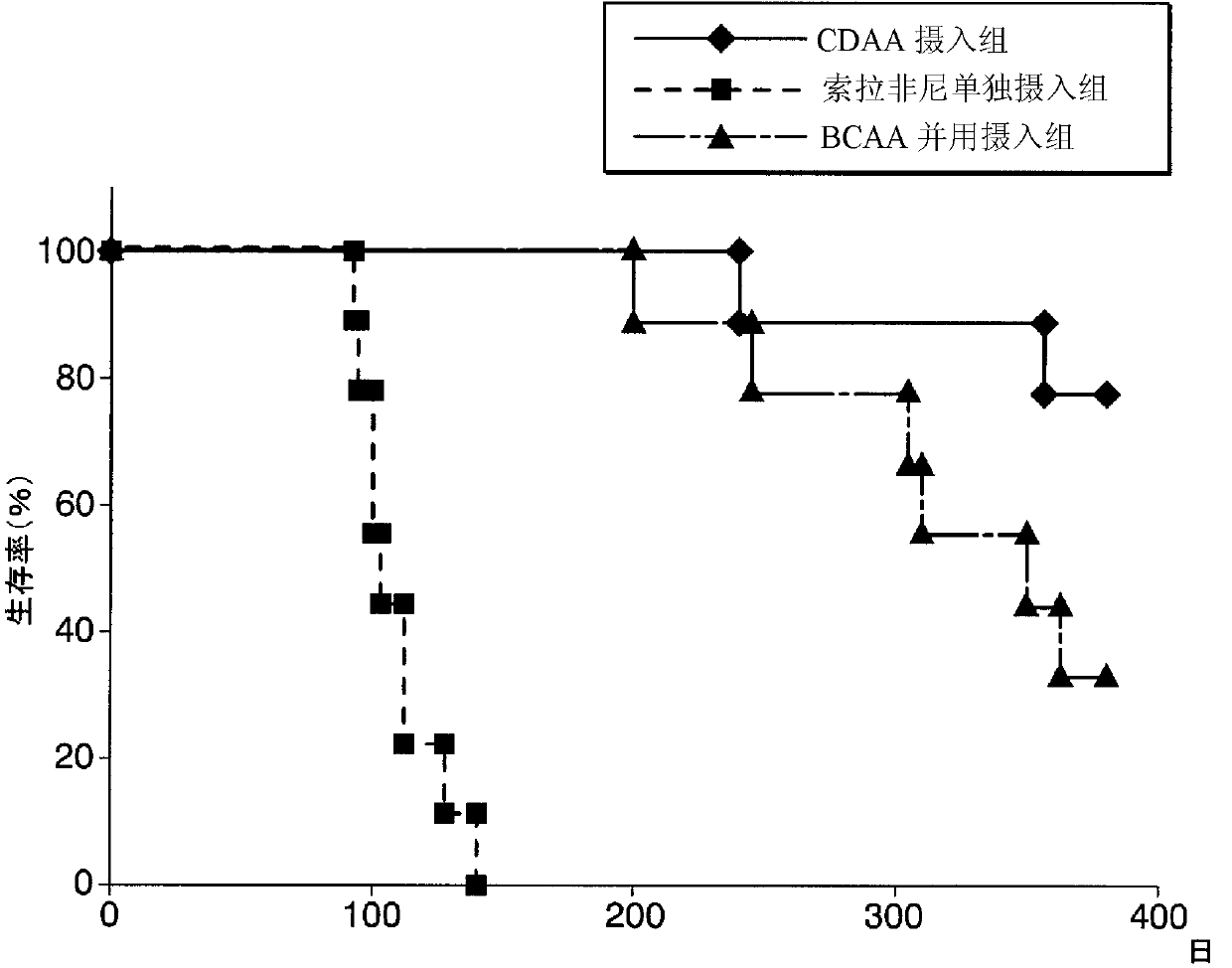
[0181] The CDAA (choline deficient amino acid supplementation) diet was used to induce liver cancer model rats, and the effect of co-administration of sorafenib and branched-chain amino acids (BCAA) was studied. As the CDAA diet, a commercially available choline-deficient diet (? 518753: Choline Deficient and iron Supplemented L-Amino Acid Defined Rat Diet, manufactured by Dyets) was used. .
[0182] Six-week-old Fischer 344 rats were divided into a CDAA dietary intake group, a sorafenib single intake group, and a BCAA combined intake group (each group: N=15), and were given the experimental diet freely. As an experimental diet, only the CDAA diet was provided to the CDAA diet intake group. For the sorafenib alone intake group, the sorafenib toluenesulfonate was mixed in the CDAA diet so that the intake of sorafenib per unit weight was 16mg / kg / day (800mg / 50kg / day). Sorafenib tablets (200 mg of Nexaball (registered trademark) tablets, manufactured by Bayer Pharmaceutical Co.,...
Embodiment 2
[0188] (inhibition of cell proliferation)
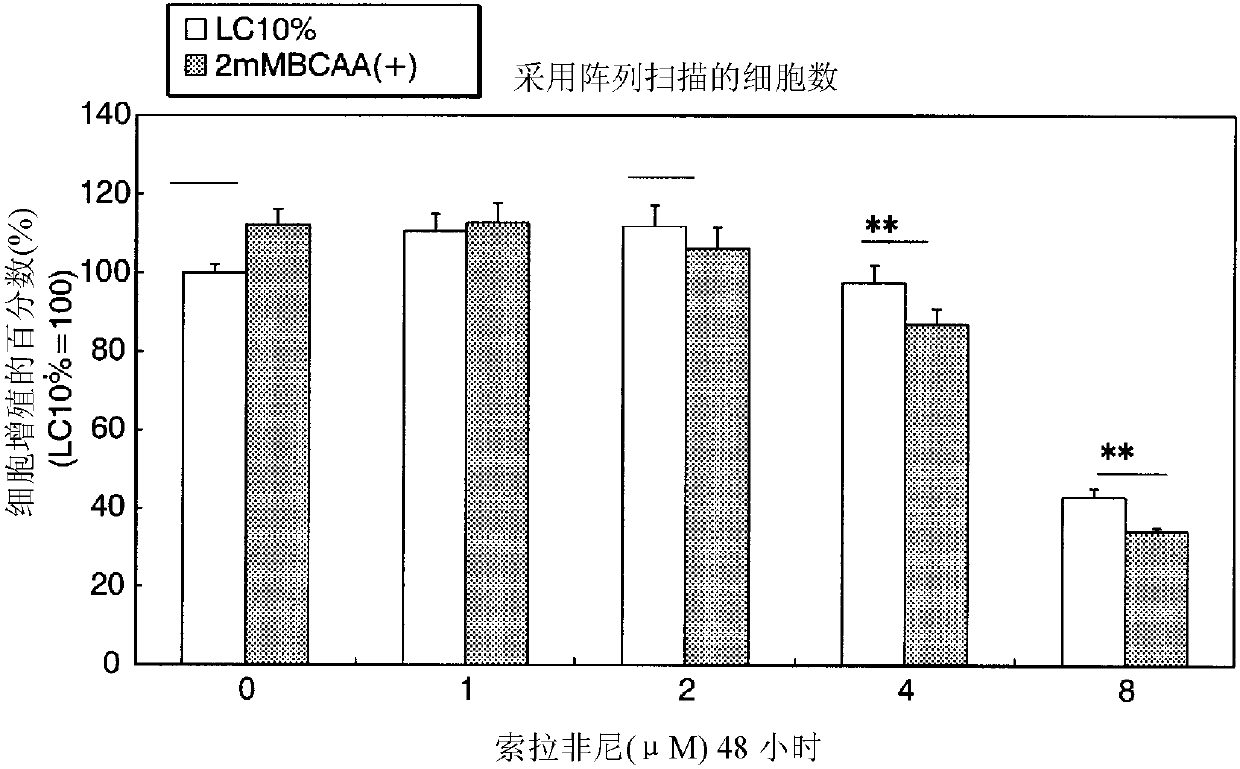
[0189] Huh7 cells were divided into 6×10 3 Cells / wells were seeded into 96-well plates, replaced with LC (containing 10% FBS) medium the next day, and 2 mM BCAA was added to Sorafenib at a concentration of 0, 1, 2, 4, and 8 μM, Incubation was carried out for 48 hours. In addition, the case of culturing for 48 hours without adding BCAA was used as a control.
[0190] After 48 hours, the medium was removed and 4% paraformaldehyde was added to the wells and fixed for 15 minutes. After removal of paraformaldehyde, 5% Hoechst solution was added to each well and left for 1 min. After removing the 5% Hoechst solution, 200 μl of PBS solution was added to each well.
[0191] The number of cells on this plate was analyzed by array scan, and the number of cells in each group was calculated as the percentage of proliferation (% of proliferation) when the control (LC10%) was set to 100. The results are shown in figure 2 .
[0192] Depend ...
Embodiment 3
[0194] (anti-tumor effect enhancing effect)
[0195] Huh7 cells in 1x10 7 Cells / mouse were subcutaneously transplanted into BALB / c nude mice.
[0196] After 1 week, they were grouped by tumor diameter, and 5 mg / kg Sorafenib was administered orally for 2 weeks (5 days / week of Sorafenib administration). Feed supply containing 3% BCAA.
[0197] The third week of administration is to increase the dosage of Sorafenib from 5mg / kg to 30mg / kg, and administer it orally for 5 days. Meanwhile, the feed containing 3% BCAA was continuously supplied.
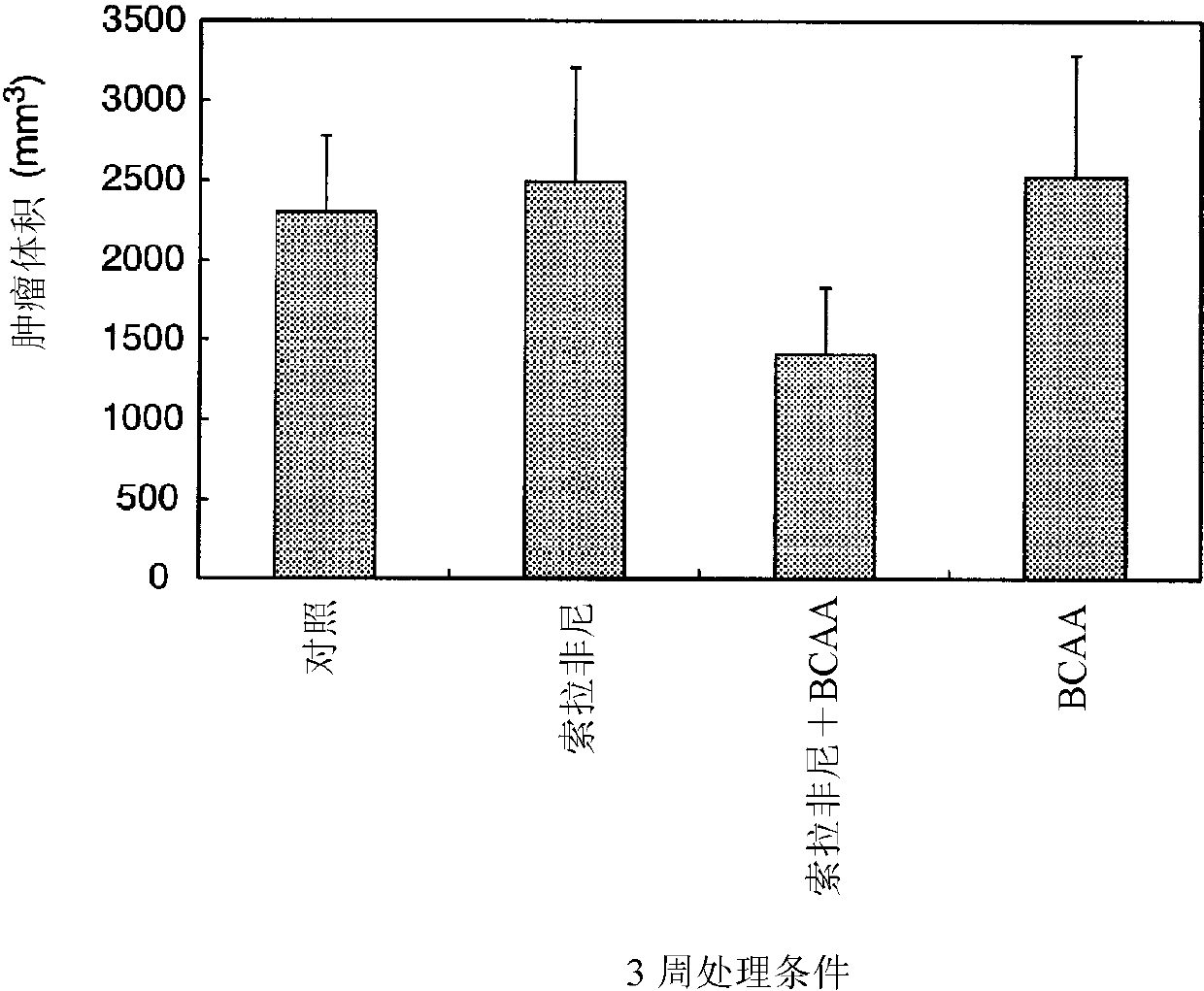
[0198] The tumor diameter of each group was measured from the start of administration to 3 weeks after, and the mean value + SE of the tumor volume was calculated. The tumor volume is based on the long diameter of the tumor / 2x (short diameter) 2 to figure it out.
[0199] The results are shown in image 3 .
[0200] Depend on image 3 From the results, it can be clearly seen that in the sorafenib + BCAA group, the degree of reduction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com