Organo-boron group-containing phosphorescent organic electroluminescent material and preparation method thereof
An organic boron group, electroluminescence technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problem of reducing the luminous efficiency of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
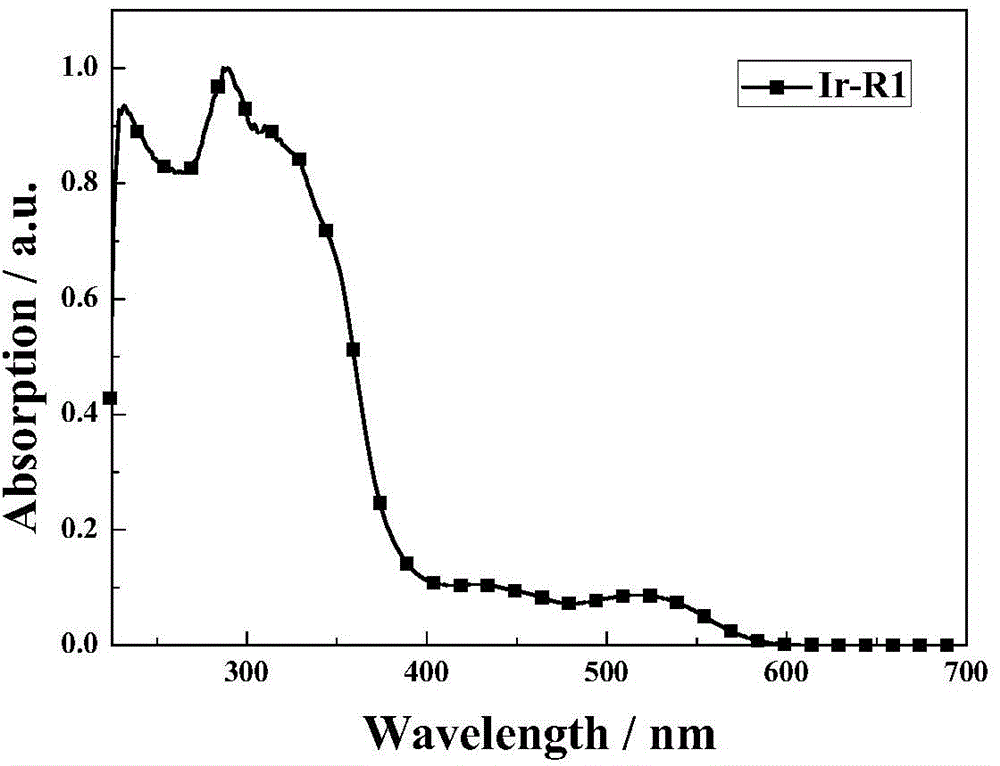
[0041] The synthesis of high-efficiency red photophosphorescent material Ir-R1, its structural formula is as follows:
[0042]
[0043] The preferred reaction process of the present embodiment is shown in the following formula:
[0044]
[0045] The specific operation process is as follows:
[0046] The first step: the present invention has no special limitation on the synthesis scheme of the organic ligand in the group, and the preferred scheme of the present invention includes the following three steps:
[0047] (1) Under nitrogen atmosphere, 2.97g (10mmol) 1-1, 2.92g (12.5mmol) pinacol ester of diboronic acid, 0.367g (0.5mmol) Pd(dppf)Cl 2 and 2.95g (30mmol) of potassium acetate (KOAc) were added into 50mL of degassed 1,4-dioxane, the reaction system was closed and the temperature was raised to 100°C for 12 hours. After the reaction system dropped to room temperature, the mixed solution obtained after the reaction was filtered under reduced pressure, and the filter ...
Embodiment 2
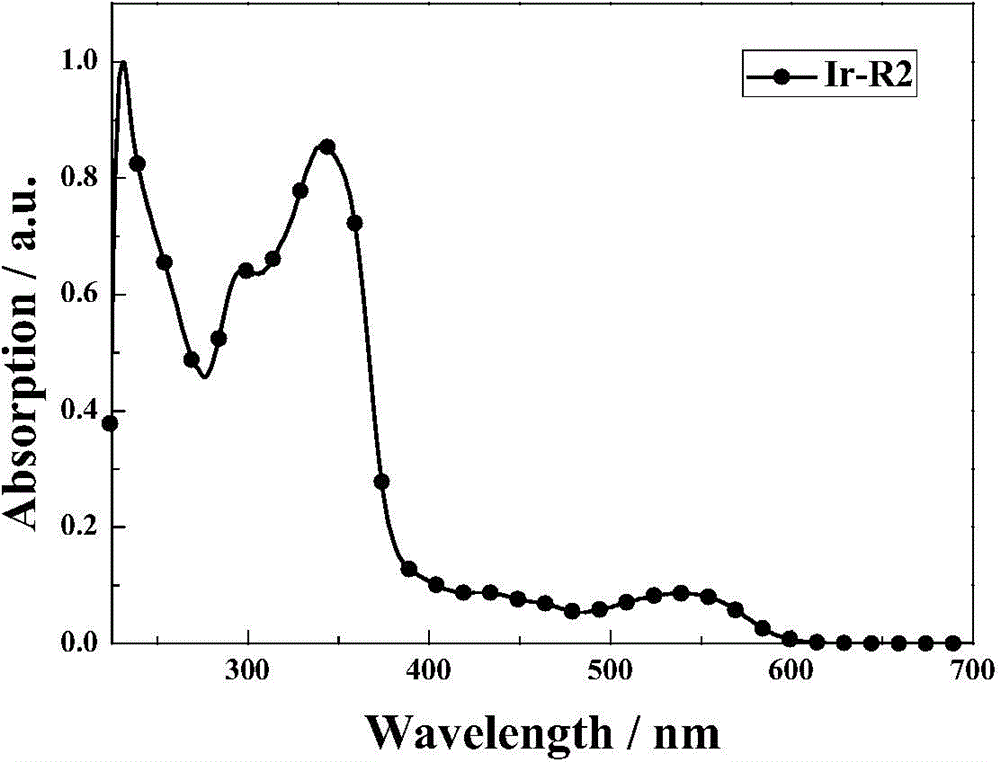
[0054] The synthesis of high-efficiency red photophosphorescent material Ir-R2, its structural formula is as follows:
[0055]
[0056] The preferred reaction process of the present embodiment is shown in the following formula:
[0057]
[0058] The specific operation process is as follows:
[0059] The first step: the present invention has no special limitation to the synthetic scheme of organic ligand in the group, and the preferred scheme of the present invention comprises following three steps:
[0060] (1) Under a nitrogen atmosphere, 4.15g (10mmol) 2-1, 2.92g (12.5mmol) pinacol borate, 0.367g (0.5mmol) Pd(dppf)Cl 2 and 2.95g (30mmol) of potassium acetate (KOAc) were added into 50mL of degassed 1,4-dioxane, the reaction system was closed and the temperature was raised to 100°C for 12 hours. After the reaction system dropped to room temperature, the mixed solution obtained after the reaction was filtered under reduced pressure, and the filter residue was washed sever...
Embodiment 3
[0068] Synthesis of high-efficiency green light-emitting phosphorescent material Ir-G, whose structural formula is as follows:
[0069]
[0070] The preferred reaction process of the present embodiment is shown in the following formula:
[0071]
[0072] The specific operation process is as follows:
[0073] The first step: the present invention has no special limitation on the synthesis scheme of the organic ligand in the group, and the preferred scheme of the present invention includes the following two steps:
[0074] (1) Under a nitrogen atmosphere, add 0.96g (3.40mmol m-bromoiodobenzene and 30mL degassed dry tetrahydrofuran) to three empty bottles, and use a liquid nitrogen / acetone bath to lower the temperature of the reaction system to -78°C. Use a syringe Take 1.6mL of 2.4M n-butyllithium n-hexane solution, slowly add it dropwise to the above reaction system, control the drop rate to 60 drops per minute, and keep it at -78°C for 45 minutes. Under nitrogen atmosph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum emission wavelength | aaaaa | aaaaa |
| Maximum emission wavelength | aaaaa | aaaaa |
| Maximum emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com