Synthesis method for low-temperature manganese-based compound metal oxide denitration catalysts
A technology of denitrification catalyst and composite metal, which is applied in the field of synthesis of low-temperature manganese-based composite metal oxide denitrification catalyst, and achieves the effects of wide application range, easy availability of raw materials, and easy control of operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
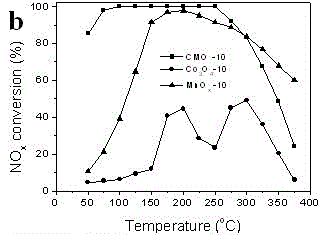
[0034] Preparation of cobalt-manganese composite oxide (CMO-10): Dissolve cobalt acetate tetrahydrate (0.0067mol) and manganese chloride tetrahydrate (0.0133mol) in 60ml of ethylene glycol, stir and cool down to - 10 oC , stabilized at this temperature for 30 min, and then slowly added an aqueous solution of sodium carbonate (200 ml, 0.2 M) dropwise at a rate of 1.2 ml / min. After the dropwise addition, the resulting precipitation system was further aged for 1 h, filtered, and then the precipitate was washed with deionized water and centrifuged for 60 o C to dry the sediment, and then at 450 o Cobalt manganese oxide (CMO-10) was obtained by calcination under C air atmosphere for 4h. A single pure manganese phase (MnO x -10) and pure cobalt phase oxides (Co 3 o 4 -10), specifically: replacing the mixture of cobalt acetate tetrahydrate and manganese chloride tetrahydrate in the above cobalt manganese oxide CMO-10 preparation method with 0.02mol manganese chloride tetrahydra...
Embodiment 2
[0038] Preparation of iron-manganese composite oxide (Fe-Mn-O-10): ferrous chloride tetrahydrate (0.0067mol) and manganese chloride tetrahydrate (0.0133mol) were dissolved in 60ml of ethylene glycol, stirred under nitrogen atmosphere and cool down to - 10 o C, stabilize at this temperature for 30 minutes, then slowly add aqueous sodium carbonate solution (200ml, 0.2M) dropwise at a rate of 1.2ml / min. After the dropwise addition, the resulting precipitation system was further aged for 1 h, filtered, and then the precipitate was washed with deionized water and centrifuged for 60 o C to dry the sediment, and then at 450 o Calcined under C air atmosphere for 4h to obtain iron manganese oxide (Fe-Mn-O-10). Pure iron phase oxides (Fe 2 o 3 -10) Catalyst, the specific method is as follows: replace the mixture of ferrous chloride tetrahydrate and manganese chloride tetrahydrate in the preparation method of iron manganese oxide (Fe-Mn-O-10) with 0.02mol ferrous chloride tetrahydra...
Embodiment 3
[0042] Preparation of nickel-manganese composite oxide (Ni-Mn-O-10):
[0043] Nickel acetate tetrahydrate (0.0067mol) and manganese chloride tetrahydrate (0.0133mol) were dissolved in 60ml of ethylene glycol, stirred and cooled to - 10 o C, stabilize at this temperature for 30 min, then slowly add aqueous sodium carbonate solution (200 ml, 0.2 M) dropwise at a rate of 1.2 ml / min. After the dropwise addition, the resulting precipitation system was further aged for 1 h, filtered, and then the precipitate was washed with deionized water and centrifuged for 60 o C to dry the sediment, and then at 450 o C calcination under air atmosphere for 4h to obtain nickel manganese oxide (Ni-Mn-O-10). The pure nickel phase oxide (NiO-10) catalyst was prepared by the same method, and the specific method was as follows: 0.02mol nickel acetate tetrahydrate was used to replace nickel acetate tetrahydrate in the preparation method of nickel manganese oxide (Ni-Mn-O-10) And the mixture of manga...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com