Compound pharmaceutical composition containing desloratadine citrate disodium and dimemorfan phosphate
A technique of dimethylorphane phosphate and its composition, which is applied in the field of medicine, can solve the problems of easy addiction to pseudoephedrine, unsuitable driving, low curative effect and side effects, and achieve the effects of no constipation side effects, less adverse reactions, and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
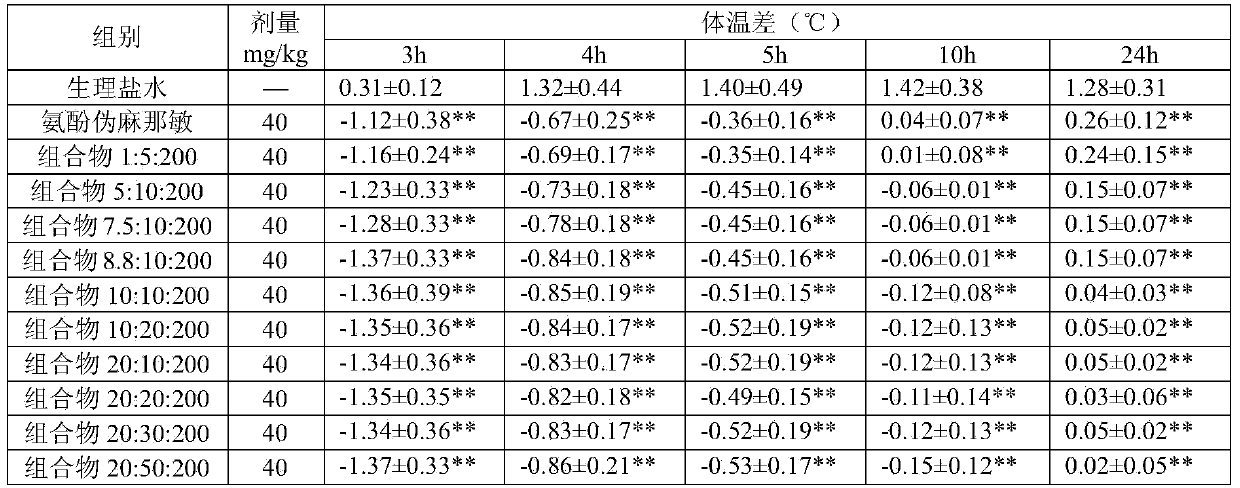
[0043] Desloratadine Citrate+Dimethorphin Phosphate+Acetaminophen Granules (according to 1000 bags)
[0044] components
Dosage
desloratadine citrate
8.8g
Dimethyphane Phosphate
10.0g
200.0g
189.0g
20.0g
Carmine
0.02g
1g
essence
4g
[0045] Preparation:
[0046] Pass desloratadine citrate, dimethylorphane phosphate, acetaminophen, lactose, and aspartame through a 80-mesh sieve, mix well, dissolve carmine in water, make a soft material, and granulate with a 20-mesh sieve , dried, granulated through a 18-mesh sieve, added essence and magnesium stearate, mixed evenly, and then divided into bags.
Embodiment 2
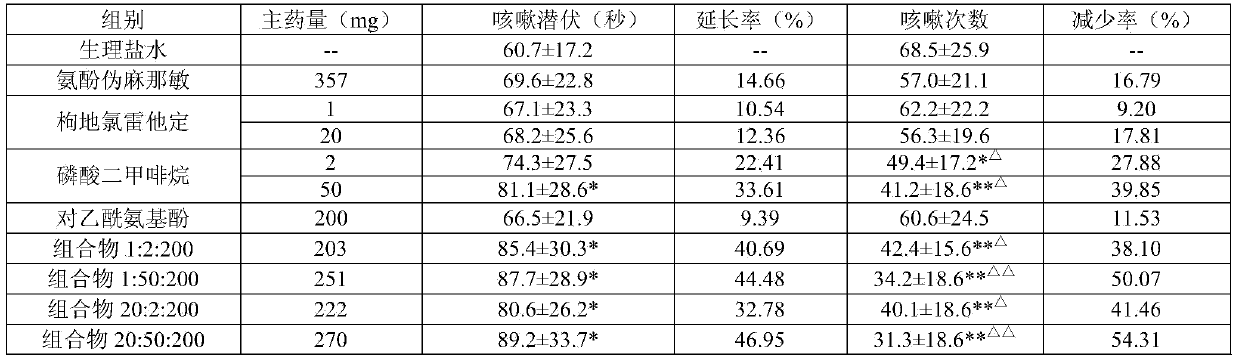
[0048] Desloratadine Citrate+Dimethorphine Phosphate+Acetaminophen Tablets (Based on 1000 tablets)
[0049] components
Dosage
desloratadine citrate
17.6g
Dimethyphane Phosphate
10.0g
Acetaminophen
200.0g
189.0g
20.0g
Carmine
0.02g
1.0g
essence
4.0g
[0050] Preparation:
[0051] Pass desloratadine citrate, dimethylmorphan phosphate, acetaminophen, microcrystalline cellulose, and aspartame through 80-mesh sieve respectively, mix well, dissolve carmine in water, and make soft material, 20-mesh Sieve and granulate, dry, granulate with 18-mesh sieve, add essence and magnesium stearate, mix evenly, and press into tablets.
Embodiment 3
[0053] Desloratadine citrate + dimethylorphine phosphate + acetaminophen solution (according to 1000 bottles)
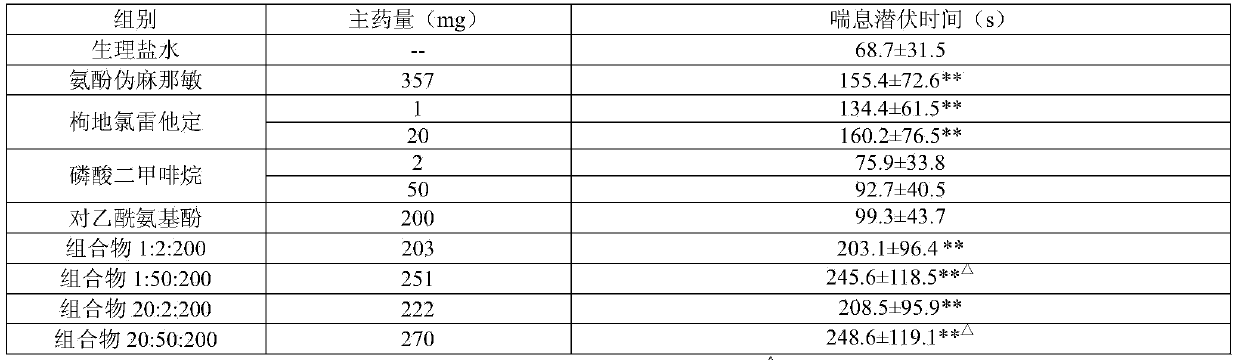
[0054] components
Dosage
desloratadine citrate
35.2g
Dimethyphane Phosphate
30.0g
Acetaminophen
200.0g
sodium benzoate
3.0g
aspartame
20.0g
2.0g
essence
4.0g
water
10000ml
[0055] Preparation:
[0056] Dissolve desloratadine citrate, dimethylorphane phosphate, and acetaminophen in an appropriate amount of water, heat and stir to dissolve, add sodium benzoate, aspartame, sodium bisulfite and essence, filter and add water to the prescribed amount , stir well and divide into packages.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com