Method for taking super-paramagnetism nanometer particles as solid phase to perform peptide synthesis and synchronously construct polypeptide magnetic nanometer probe
A technology for synthesis of nanoparticles and peptides, which is applied in peptide preparation methods, chemical instruments and methods, biochemical equipment and methods, etc., and can solve problems such as difficulty in obtaining peptide sequences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
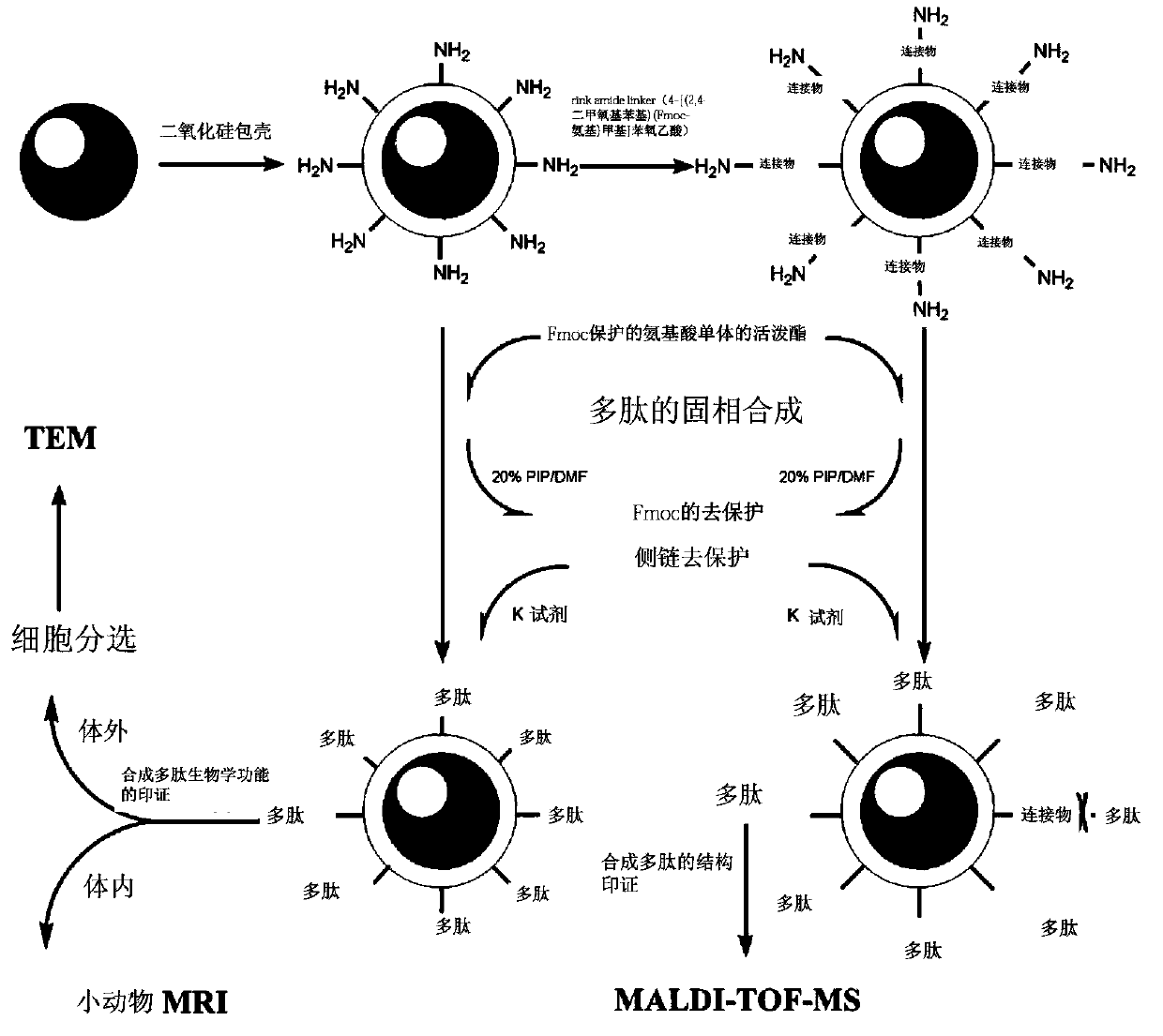
[0075] Embodiment 1 uses superparamagnetic Fe 3 o 4 Nanoparticle-based solid-phase synthesis of RGDGG pentapeptide and simultaneous construction of RGDGG-magnetic nanoprobes
[0076] Schematic such as figure 1 shown.
[0077] 1. Preparation of silica-coated superparamagnetic Fe 3 o 4 Nanoparticles (prepared according to published methods: Monodisperse MFe 2 o 4 (M)Fe,Co,Mn) Nanoparticles, Shouheng Sun, et.al, J.AM.CHEM.SOC.2004,126,273-279. ) and coupled rink amide linker (4-[(2,4-dimethoxyphenyl)(Fmoc-amino)methyl]phenoxyacetic acid, purchased from Jill Biochemical Shanghai Co., Ltd.) Silica coating superparamagnetic Fe 3 o 4 Nanoparticle solid phase:
[0078] Silica-coated superparamagnetic Fe 3 O 4 Preparation of Nanoparticle Solid Phase:
[0079] 1) Prepare 100ml of superparamagnetic Fe with a concentration of 0.05mg / ml 3 o 4 Chloroform solution A of nanoparticles;
[0080] 2) Place solution A at 40°C, and concentrate the solution to 10 microliters...
Embodiment 2
[0113] Embodiment 2 uses superparamagnetic Fe 2 o 3 Nanoparticle-based solid-phase synthesis of RGDGG pentapeptide and simultaneous construction of RGDGG-magnetic nanoprobes
[0114] According to the method for embodiment 1, prepare the superparamagnetic Fe of silica coating 2 o 3 Silica coating of nanoparticles and coupling rinkamide linker (4-[(2,4-dimethoxyphenyl)(Fmoc-amino)methyl]phenoxyacetic acid, purchased from Jill Biochemical Shanghai Co., Ltd.) superparamagnetic Fe 2 o 3 nanoparticles. Using the two as solid-phase synthesis of RGDGG pentapeptide and synchronous construction of RGDGG-magnetic nanoprobes, the method is the same as in Example 1.
Embodiment 3
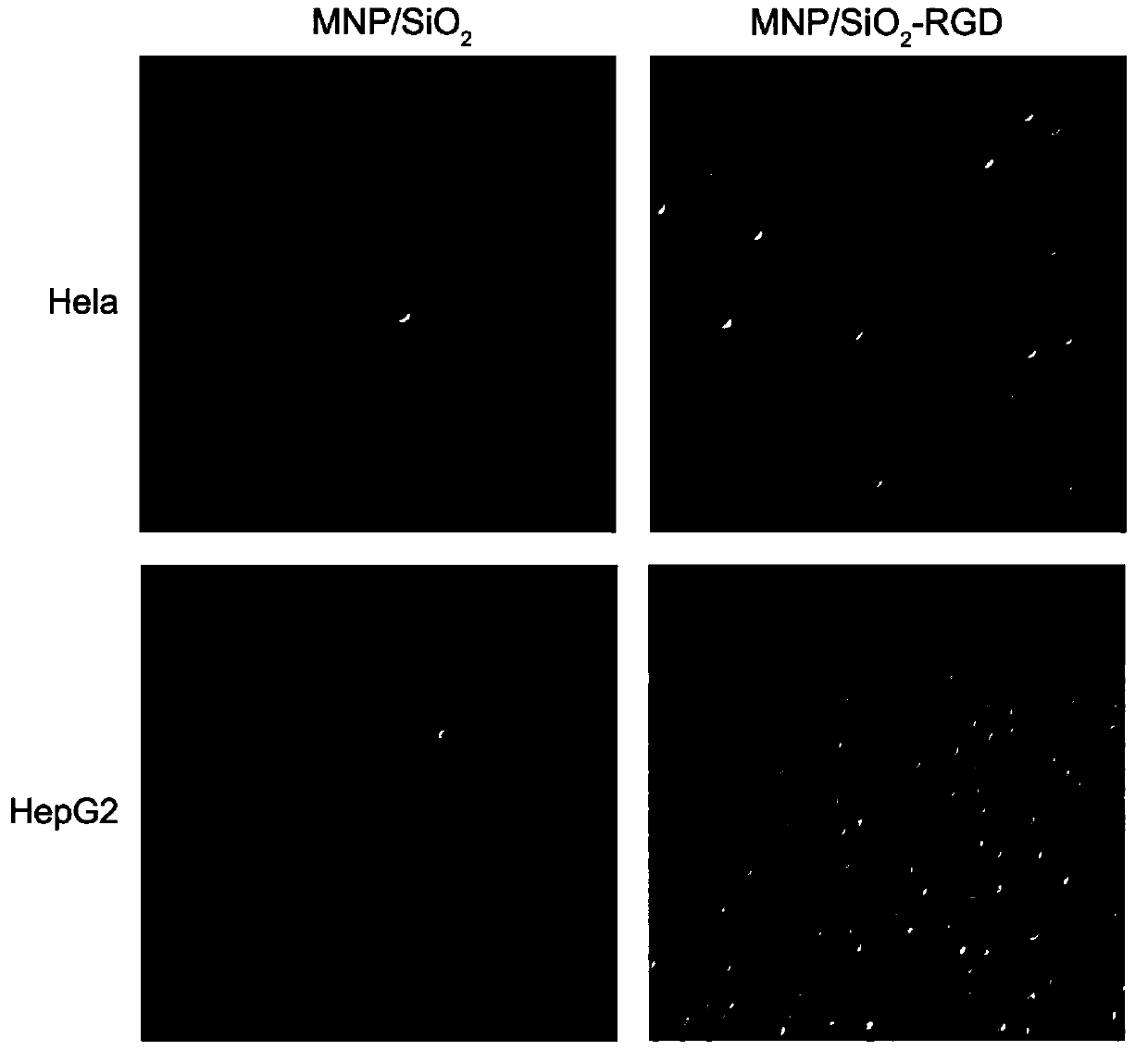
[0115] Example 3 In Vitro Cell Sorting Experiment
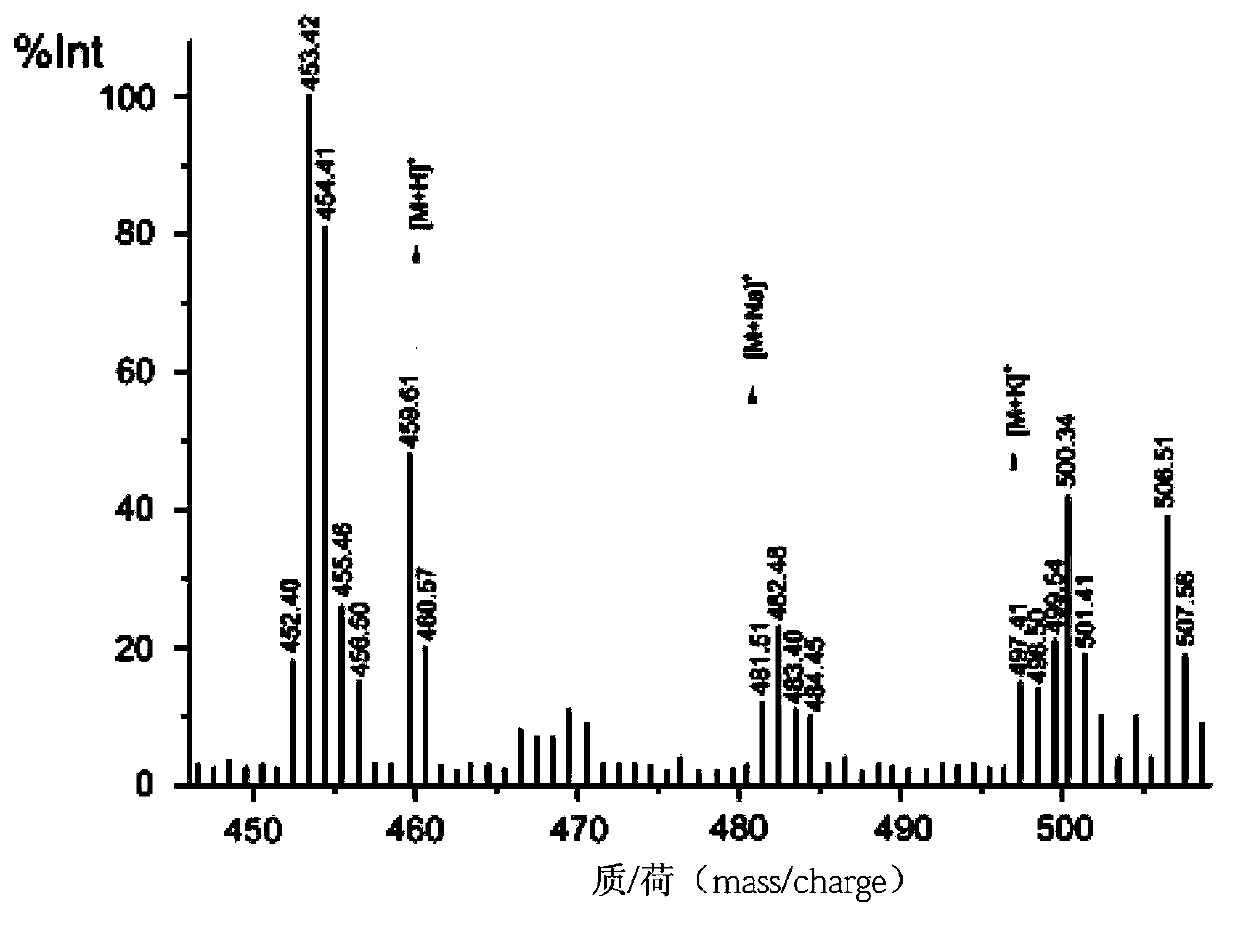
[0116] The peptide-conjugated magnetic nanoprobe obtained from the solid phase of superparamagnetic nanoparticles coated with silica in step 3b of Example 1 was dissolved in PBS, and the effect of the synthetic peptide on cells was confirmed by in vitro cell sorting experiments.
[0117] method:
[0118] 1) Add the peptide magnetic probe (0.15mg) obtained from the solid phase of superparamagnetic nanoparticles coated with silica into 1ml of Hela cell and HepG2 cell suspension (the cell concentration is 200,000 cells / ml), and place in a rotating Incubate at 4°C for 2 hours in a mixer;
[0119] 2) Place the incubated two groups of cells on a magnet for 5 minutes, discard the solution, and wash three times with PBS;
[0120] 3) The suspension obtained after the treatment in step 2) is observed under a light microscope, and the strength of the polypeptide's effect on the cells can be judged by the number of cells in the field o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com