Application of physagulin P in preparation of antitumor drug
An anti-tumor drug, the technology of picrolactone, which is applied in the field of anti-tumor drugs, can solve the problems of far-reaching differences in cancer treatment, and achieve the effects of significant cell inhibition, inhibition of growth and proliferation, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation process of STAT3 inhibitor picrolactone P with anti-tumor activity.
[0032] 1) Put 1Kg of the persistent calyx of Phyllostachys chinensis into the extraction tank, add 95% ethanol aqueous solution (i.e. 5Kg of ethanol aqueous solution) containing 5 times of the weight of the persistent calyx of Phyllostachys dioica, each time, and extract under reflux at 90°C, The time of each extraction is 2h, a total of 4 extractions (ethanol aqueous solution totals 20Kg), and the combined extracts;
[0033]2) Concentrate the extract obtained in step 1) (recover ethanol) to nearly dryness to obtain 130 g of thick extract, which is diluted with 1 L of water and extracted with 1 L of ethyl acetate each time for a total of 3 extractions, and the extracts are combined;
[0034] 3) 20 g of the extract obtained by concentrating the extract obtained in step 2) is passed through a silica gel chromatographic column (wet column loading, dry loading), wherein the filler u...
Embodiment 2
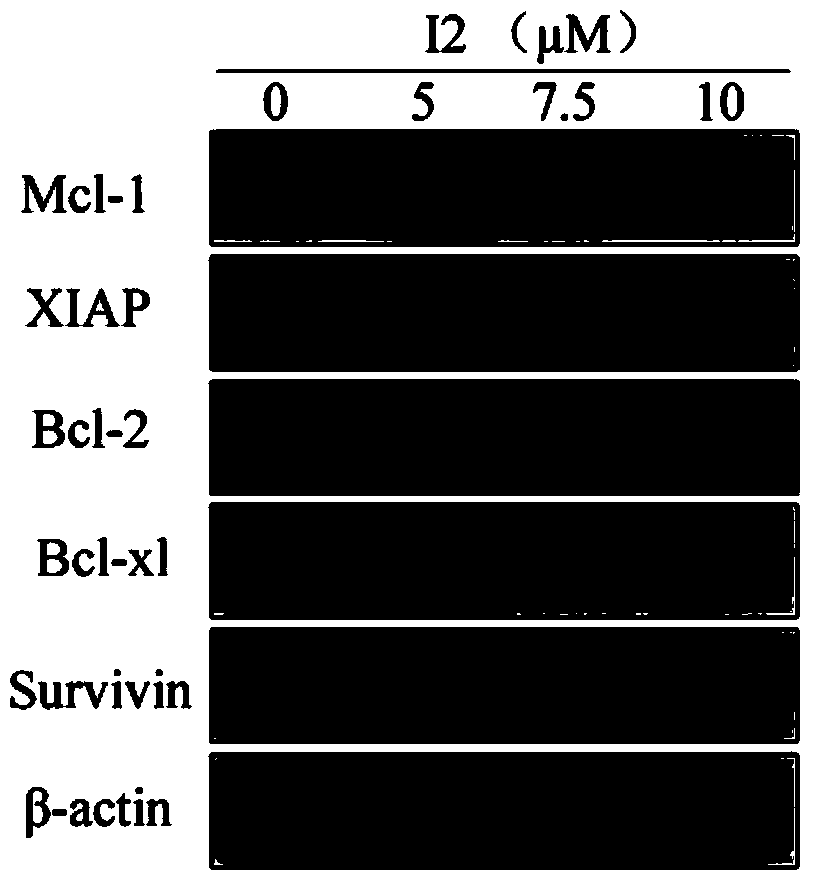
[0037] Example 2: Kumarin P inhibits the phosphorylation level of Tyr705 (Tyr705) of STAT3 kinase in human gastric cancer cell HGC27, and its inhibitory effect decreases in a concentration-dependent manner.
[0038] experimental method:
[0039] Human gastric cancer cells HGC27 were grown in RPMI1640 medium containing 10% (wt) FBS (GIBCO) at 37°C in 5% CO 2 Incubator (Thermo) cultivation. When the cells grow to 70%-80%, intervene the cells with different concentrations of picrolactone P (0, 5 μM, 7.5 μM, 10 μM). After 4 hours of drug treatment, collect the cells, discard the supernatant, and add 1ml 4°C The cells were washed with pre-cooled 1×PBS (pH7.4±0.2), and centrifuged at 8000 rpm for 5 minutes. Discard the supernatant, add 0.1ml RIPA lysate, and lyse the cells on ice for 30 minutes, shaking every 10 minutes. After the cells are fully lysed, centrifuge the lysate at 8000rpm for 10 minutes, transfer the supernatant to another 1.5ml Eppendorf tube, measure the protein c...
Embodiment 3
[0041] Example 3: Effects of picrolactone P on the phosphorylation levels of Src and JAK2 kinases and the expression levels of phosphatases SHP1 and SHP2 in human gastric cancer cell HGC27.
[0042] Experimental method: with embodiment 2, its detection result is as figure 2 shown.
[0043] Such as figure 2 As shown, different concentrations of picrolactone P acted on HGC27, and it had no significant effect on the phosphorylation levels of kinases Src and JAK2 upstream of STAT3 and the expression levels of phosphatases SHP1 and SHP2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com