Synthetic method for intermediate of benserazide hydrochloride
A technology of benserazide hydrochloride and a synthesis method, which are applied in directions such as hydrazone preparation and organic chemistry, can solve problems such as long production cycle and low yield, and achieve the effects of shortening production cycle, improving product yield and convenient handling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
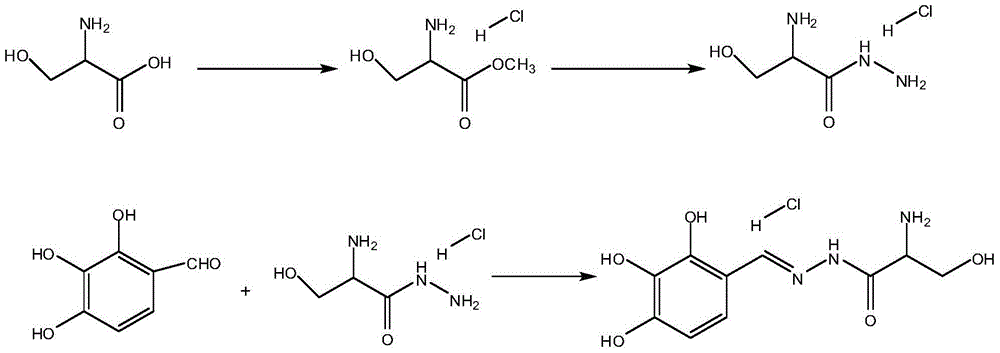
[0033] A kind of synthetic method of the intermediate of benserazide hydrochloride, comprises the steps:
[0034] Step S1: Add 300ml of methanol to a 500ml four-neck flask, cool down to 15°C; add 55g of thionyl chloride dropwise to methanol, add 50g of DL-serine, and reflux for 4 hours, and place the reaction solution in a water bath at 45°C Concentrate to dryness under reduced pressure and set aside.
[0035] Step S2: Preparation of Serine Hydrazide:
[0036] Add 95g of hydrazine hydrate into a 500ml four-neck bottle, dissolve the serine methyl ester prepared in Test 1 with an appropriate amount of methanol, add the serine methyl ester dropwise to the hydrazine hydrate at 20°C, after the drop is complete, keep the reaction at 20°C for one hour, and cool to At room temperature, adjust the pH to 4.0-5.0 with concentrated hydrochloric acid, add 100ml of isopropanol, cool to 10°C, stir and crystallize for 3 hours; filter to obtain the product.
[0037] Step S3: Preparation of B...
Embodiment 2
[0041] A kind of synthetic method of the intermediate of benserazide hydrochloride, comprises the steps:
[0042] Step S1: Add 315ml of methanol into a 500ml four-neck flask, cool down to 10°C; add 60g of thionyl chloride dropwise into methanol, add 50g of DL-serine, reflux for 3 hours, and concentrate the reaction solution under reduced pressure to Dried and set aside.
[0043] Step S2: Preparation of Serine Hydrazide:
[0044] Add 100g of hydrazine hydrate into a 500ml four-necked bottle, dissolve the serine methyl ester prepared in step S1 with an appropriate amount of methanol, add the serine methyl ester dropwise to the hydrazine hydrate at 25°C, after the drop is complete, keep the reaction at 25°C for one hour, and cool to At room temperature, adjust the pH to 4.0-5.0 with concentrated hydrochloric acid, add 100ml of n-butanol, cool to 12°C, stir and crystallize for 3 hours; filter to obtain the product.
[0045] Step S3: Preparation of Benzylsilazone Hydrochloride: ...
Embodiment 3
[0049] A kind of synthetic method of the intermediate of benserazide hydrochloride, comprises the steps:
[0050] Step S1: Add 350ml of methanol to a 500ml four-necked flask, cool down to 5°C; add 65g of thionyl chloride dropwise to methanol, add 50g of DL-serine, reflux for 5 hours, and concentrate the reaction solution under reduced pressure to Dried and set aside.
[0051] Step S2: Preparation of Serine Hydrazide:
[0052] Add 105g of hydrazine hydrate into a 500ml four-necked bottle, dissolve the serine methyl ester prepared in step S1 with an appropriate amount of methanol, add the serine methyl ester dropwise to the hydrazine hydrate at 30°C, after the drop is complete, keep the reaction at 30°C for one hour, and cool to At room temperature, adjust the pH to 4.0-5.0 with concentrated hydrochloric acid, add 100ml of ethanol, cool to 15°C, stir and crystallize for 3 hours; filter to obtain the product.
[0053] Step S3: Preparation of Benzylsilazone Hydrochloride:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com