Herbicide composition and application thereof
A composition and herbicide technology, applied in the direction of herbicide and algicide, application, biocide, etc., to achieve the effect of expanding the herbicide spectrum and improving the control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0246] Embodiment 1 (34.5% wettable powder)
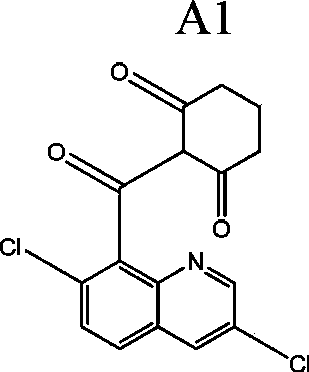
[0247] Bensulfuron-methyl 4.5%, 2-(quinolin-8-yl)-carbonyl-cyclohexane-1,3-dione compound or its tautomer 30%, sodium dodecylbenzenesulfonate 2.0%, sodium lignosulfonate 10%, diatomaceous earth to make up to 100%. In this example, the 2-(quinolin-8-yl)-carbonyl-cyclohexane-1,3-dione compound or its tautomer is specifically the compound A1 given in the specification.
Embodiment 2
[0248] Embodiment 2 (37.5% wettable powder)
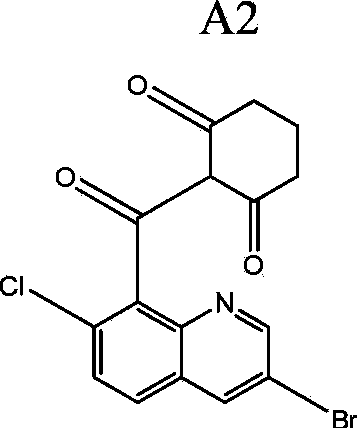
[0249] Clopyrazosulfuron 7.5%, 2-(quinolin-8-yl)-carbonyl-cyclohexane-1,3-dione compound or its tautomer 30%, sodium lauryl sulfate 1.5 %, sodium lignosulfonate 10%, kaolin to make up to 100%. In this example, the 2-(quinolin-8-yl)-carbonyl-cyclohexane-1,3-dione compound or its tautomer is specifically the compound A2 given in the specification.
Embodiment 3
[0250] Embodiment 3 (25.5% wettable powder)
[0251] Pyrazosulfuron-methyl 3%, 2-(quinolin-8-yl)-carbonyl-cyclohexane-1,3-dione compound or its tautomer (A3) 22.5%, alkamido taurine Salt 6%, alkylnaphthalene formaldehyde condensate 5%, sodium dodecylbenzene sulfonate 5%, kaolin to make up to 100%. In this example, the 2-(quinolin-8-yl)-carbonyl-cyclohexane-1,3-dione compound or its tautomer is specifically the compound A3 given in the specification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com