A kind of montelukast sodium granule and preparation method thereof
A technology of montelukast sodium and granules, applied in the field of medicine, can solve problems such as hidden safety hazards and human harm, and achieve the effect of slowing down the impact and taking it safely.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
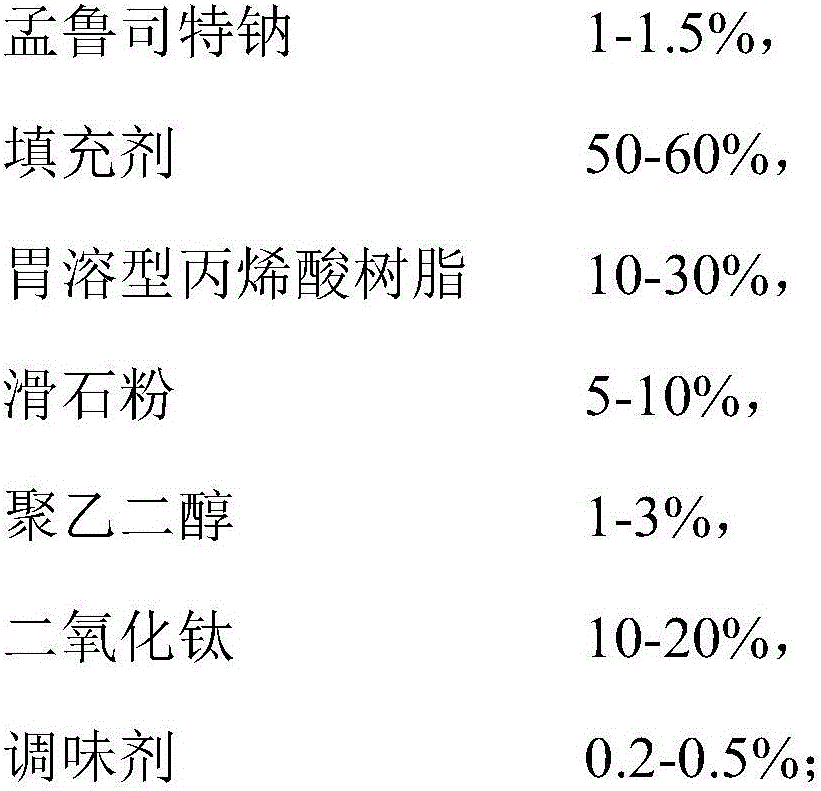
[0024]
[0025]
[0026] According to the above components, proceed as follows:
[0027] (1), stomach-soluble acrylic resin E (PO), titanium dioxide, talcum powder, polyethylene glycol are made into 10% gastric-soluble acrylic resin ethanol solution;
[0028] (2) After the raw materials are evenly mixed with mannitol and microcrystalline cellulose, use 1 / 3 of 10% gastric-soluble acrylic resin ethanol liquid to make a soft material, centrifugally spheronize, and dry at 40 OC to obtain particles below 60 mesh;
[0029] (3), the particles are placed in a fluidized bed, coated with 2 / 3 of 10% gastric-soluble acrylic resin coating liquid, and dried at 40°C for 2 hours;
[0030] (4), take the dried granules, add strawberry essence and aspartame and mix evenly;
[0031] (5) After the content was determined, the finished product 1 was obtained by dispensing according to the specification of 0.5 g containing 4 mg of montelukast.
Embodiment 2
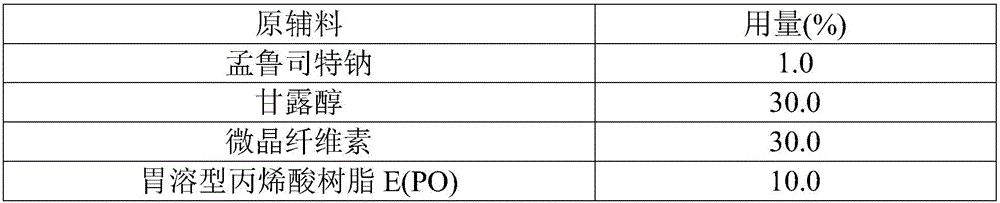
[0033] Raw materials
[0034] According to the above formula, adopt the same preparation method as in Example 1, the difference is only in the replaced components, so as to obtain the finished product 2.
Embodiment 3
[0036]
[0037]
[0038] According to the above formula, adopt the same preparation method as in Example 1, the difference is only in the replaced components, so as to obtain the finished product 3.
[0039] According to above embodiment, carry out test, specifically as follows:
[0040] Stability research between the present invention and commercially available samples
[0041]
[0042] It can be seen from the above results that the stability of the product of the present invention is much stronger than that of the commercially available samples, and it is safer to use.
[0043] The release degree of the present invention and commercially available samples in artificial gastric juice
[0044] sampling time
[0045] As can be seen in this table, its release is stable and easy to absorb.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com