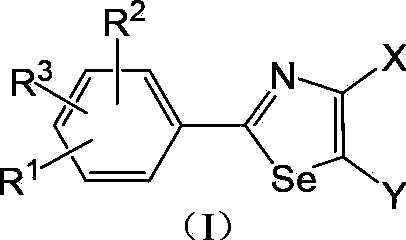
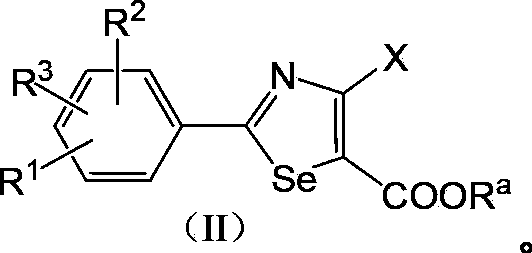
2-Arylselenazole compounds and medicinal composition thereof
A compound, the technology of selenazole, which is applied in the field of xanthine oxidase inhibitors to treat gout and hyperuricemia, 2-aryl selenazole compounds, can solve the problems of large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Synthesis of 2-(3-cyano-4-ethoxyphenyl)-4-methyl-selenazole-5-carboxylic acid (6)
[0146]
[0147] Step A: Add absolute ethanol (540mL) dropwise to a mixture of selenium powder (50.0g, 0.633mol) and sodium borohydride (26.4g, 0.698mol) within 3 to 4 hours under ice-water bath and nitrogen protection, After the addition was complete, the temperature was raised to room temperature and stirring was continued for 1 hour. Then add a pyridine solution (126mL) containing p-hydroxybenzonitrile (18.84g, 0.158mol), raise the temperature to reflux, then slowly add 2M hydrochloric acid solution (320mL) dropwise, and the dropwise addition time is not less than 4 hours, and reflux after the addition is completed. overnight. TLC analysis indicated that the reaction was complete. Most of the ethanol was evaporated under reduced pressure, diluted with water (400mL), extracted with ethyl acetate (200mL×2), the combined organic phase was washed with 2M hydrochloric acid (100mL), the...
Embodiment 2
[0156] Synthesis of 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-selenazole-5-carboxylic acid (7)
[0157]
[0158] For the experimental operation, see Step E and Step F in Example 1.
[0159] 1 H NMR (DMSO-d 6 , 400MHz) δ8.26(d, J=2.4Hz, 1H), 8.18(dd, J=2.0, 9.2Hz, 1H), 7.34(d, J=9.2Hz, 1H), 4.00(d, J=6.8 Hz, 2H), 2.63(s, 3H), 2.14~2.04(m, 1H), 1.02(d, J=6.8Hz, 6H). MS (EI, m / z): 363.2 [M-H] - .
Embodiment 3
[0161] Synthesis of 2-(3-cyano-4-isopropoxyphenyl)-4-methyl-selenazole-5-carboxylic acid (8)
[0162]
[0163] For the experimental operation, see Step E and Step F in Example 1.
[0164] 1 H NMR (DMSO-d 6 , 400MHz) δ8.29(d, J=2.4Hz, 1H), 8.20(dd, J=2.4, 8.8Hz, 1H), 7.38(d, J=8.8Hz, 1H), 4.94~4.88(m, 1H ), 2.65(s, 3H), 1.36(d, J=6.0Hz, 6H). MS (EI, m / z): 349.1 [M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com