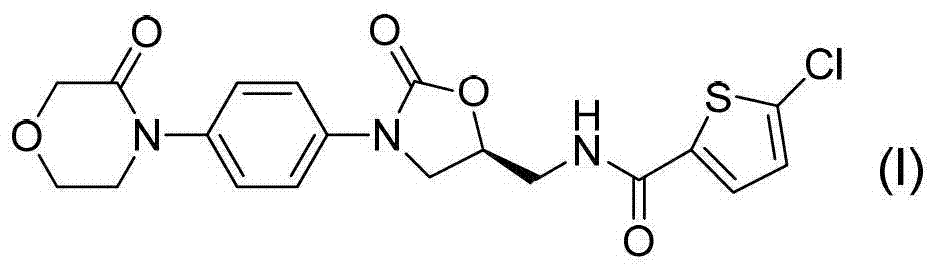
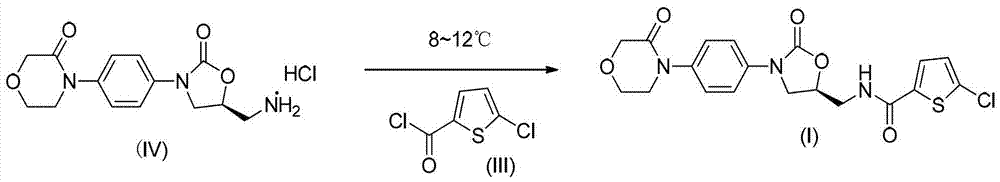
Preparation method of 5-penphene-2-formyl chloride
A technology of chlorothiophene and formyl chloride, applied in the direction of organic chemistry, can solve the problems of severe irritation to the eyes, skin and respiratory tract, high toxicity and corrosion, etc., achieve great implementation value and social economic value, less impurities, and short reaction cycle Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of 5-chlorothiophene-2-formyl chloride
[0027] Add 14.5g (0.09mol) of 5-chlorothiophene-2-carboxylic acid into a 250ml four-necked bottle, add 150ml of dichloromethane and 12.5ml (0.09mol) of triethylamine to dissolve, connect the drying device and the acid gas absorption device. Dissolve 26.7g (0.09mol) of triphosgene in 50ml of dichloromethane (1.8mol / L), drop it into a four-necked flask at room temperature, and stir and reflux for 20 hours. After the reaction, an appropriate amount of anhydrous magnesium sulfate was added to the system, stirred, suction filtered, and the mother liquor was rotary evaporated to obtain 17 g of a yellow oil, and the yield of the crude product was 100%.
Embodiment 2
[0028] Embodiment 2: Preparation of 5-chlorothiophene-2-formyl chloride
[0029] Add 14.5g (0.09mol) of 5-chlorothiophene-2-carboxylic acid into a 250ml four-necked bottle, add 150ml of dichloromethane and 7.2g (0.09mol) of pyridine to dissolve, connect the drying device and the acid gas absorption device, at room temperature Put the dichloromethane solution of triphosgene (26.7g / 50ml=1.8mol / L) dropwise into the four-neck flask, stir and reflux for 20 hours. After the reaction, an appropriate amount of anhydrous sodium sulfate was added to the system, stirred, filtered with suction, and the mother liquor was rotary evaporated to obtain 17.5 g of a yellow oil, and the yield of the crude product was 100%.
Embodiment 3
[0030] Embodiment 3: Preparation of 5-chlorothiophene-2-formyl chloride
[0031] Add 8.1g (0.05mol) of 5-chlorothiophene-2-carboxylic acid in a 250ml four-necked bottle, add 100ml of dichloromethane and 5.5ml (0.04mol) of triethylamine to dissolve, connect the drying device and the acid gas absorption device, and At 40°C, drop triphosgene in dichloromethane (26.7g / 50ml=1.8mol / L) into a four-neck flask, stir and reflux for 8 hours. After the reaction, an appropriate amount of anhydrous magnesium sulfate was added to the system, stirred, suction filtered, and the mother liquor was rotary evaporated to obtain a yellow oil and an insoluble solid (raw material). GC detected that the reaction was incomplete, and the yield was 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com