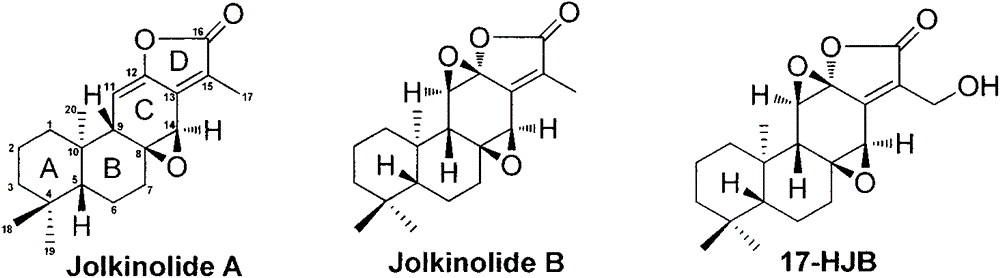
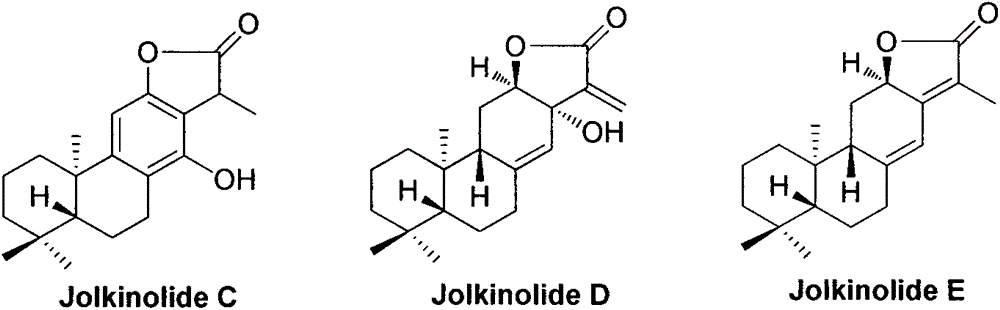
Synthesis method for natural jolkinolide A and B
A technology of spurge lactone and Euphorbia serrata, applied in the field of spurge lactone compounds, can solve the problems of low total yield, low content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
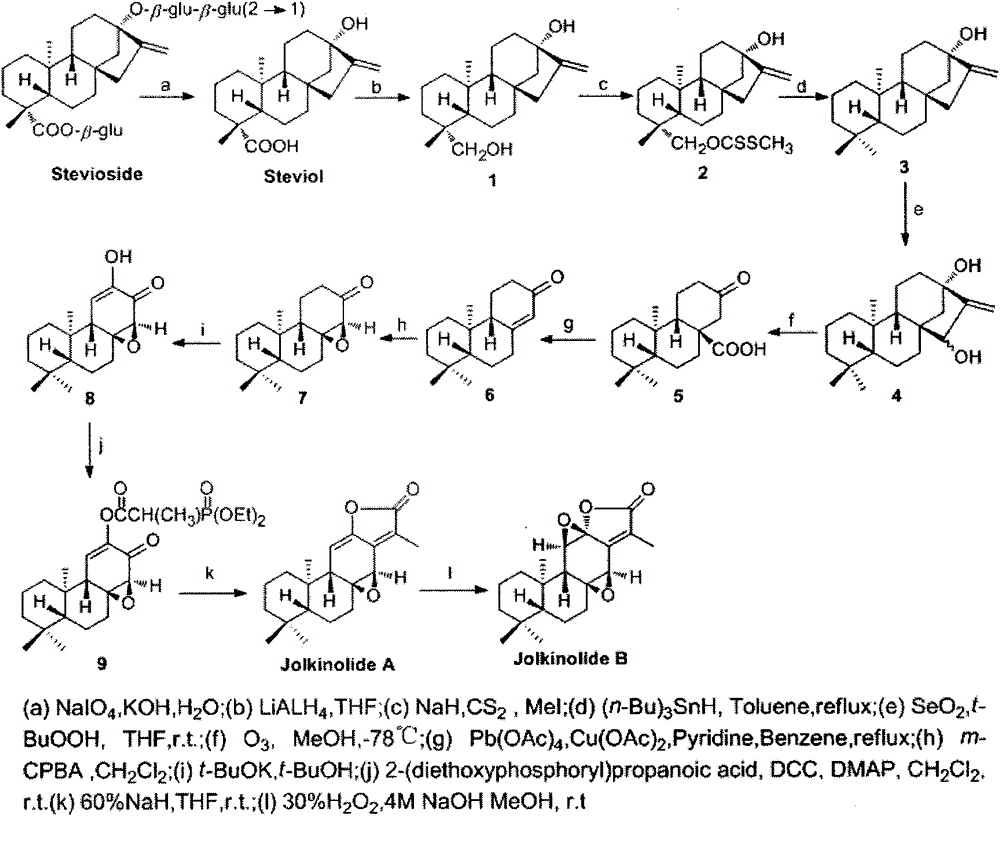
[0043] Compound 1 (2g, 6.57mmol) was put into a reaction flask, dissolved in THF (20ml), added 60% sodium hydride (0.47g, 19.71mol), heated to reflux for 3-5h, cooled to room temperature, added carbon disulfide (0.44 ml, 7.23mmol), kept at 50-60°C for 1h, cooled to room temperature, added iodomethane (0.45ml, 7.23mmol), and reacted at room temperature for 30min. Under ice-bath conditions, water was added dropwise to quench the reaction, part of the solvent was evaporated, extracted with ethyl acetate, dried, and column chromatography (petroleum ether:ethyl acetate (V / V)=10:1) gave 15.5g of white solid compound 2. The yield is 81%. mp: 103-105℃.IR(KBr,cm -1 ): 3470, 3302, 2982, 2925, 2849, 2359, 1708, 1695, 1444, 1230, 1070, 1057, 960, 874, 749; 1 H-NMR (CDCl 3 , 300MHz) δ: 4.98(s, 1H), 4.82(s, 1H), 4.76-4.76(d, J=11, 1H), 4.41-4.38(d, J=10.9, 1H), 2.56(s, 3H ), 2.18-2.10(m,3H), 1.88-1.81(m,5H), 1.77-1.73(m,3H), 1.63-1.42(m,9H), 1.26-1.22(d, J=9.5,1H) , 1.01(s, 6H). 13 C-...
Embodiment 2
[0045] Put compound 2 (3.1g, 7.86mmol) into the reaction flask, add toluene (100ml) to dissolve, add the catalyst, and use a syringe to add tributyltin hydride (3.7mL, 13.35mmol) to the reaction solution within 3h under nitrogen protection , maintain the reaction temperature at 80-100°C. Part of the solvent was evaporated, extracted with ethyl acetate, dried, and column chromatographed (petroleum ether: ethyl acetate (V / V) = 15:1) to obtain 3.9 g of white solid compound 3 with a yield of 88%. mp: 117-119℃.IR(KBr,cm -1 ): 3111, 2994, 2946, 2992, 2846, 2842, 2360, 2354, 1400, 1367, 1112, 1088,877; 1 H-NMR (CDCl 3 , 300MHz) δ: 4.98(s, 1H), 4.82(s, 1H), 2.19-2.11(m, 3H), 1.85-1.764(m, 3H), 1.66-1.65(m, 2H), 1.62-1.59( m,2H)1.58-1.49(m,6H), 1.45-1.42(m,2H), 1.39-1.30(m,2H), 1.28-1.13(m,7H), 1.03(s,3H,); 13 C-NMR (300MHz, CDCl 3 )δ: 156.3, 102.7, 80.4, 56.2, 54.7, 47.6, 47.1, 42.0, 41.7, 41.3, 40.3, 39.4, 39.1, 33.6, 33.3, 23.4, 21.2, 20.2, 18.6, 17.5; ESI-MS m / z: 311[M+Na] +...
Embodiment 3
[0047] Compound 3 (2.6g, 9.01mmol)) was put into the reaction flask, THF (30ml) was added to dissolve, selenium dioxide (0.6g, 5.41mmol) and tert-butyl hydroperoxide (1mL) were added successively, and the reaction was carried out at room temperature for 10-24h . It was diluted with water, extracted with ethyl acetate, dried, and column chromatographed (petroleum ether: ethyl acetate (V / V) = 3: 1) to obtain 32.2 g of white solid compound 4 with a yield of 80%. mp: 244-246°C; IR(KBr,cm -1 ): 3551, 3474, 3227, 3115, 2932, 1370, 1363, 1004, 562; 1 H-NMR (CDCl 3 , 300MHz) δ: 5.30(s, 1H), 5.26(s, 1H), 3.82(s, 1H), 2.10-2.06(d, J=11.1, 1H), 1.88-1.76(m, 4H), 1.75- 1.49(m, 5H), 1.44-1.37(m, 4H), 1.31-1.25(m, 3H), 1.18-1.11(m, 2H), 1.02(s, 3H), 0.95-0.92(m, 1H), 0.87(s, 3H), 0.82(s, 3H). 13 C-NMR (300MHz, CDCl 3 )δ: 161.6, 107.1, 80.8, 78.1, 56.2, 53.5, 45.6, 43.4, 42.0, 40.8, 40.5, 39.1, 35.5, 33.9, 33.3, 21.9, 19.9, 19.5, 18.6, 17.8; ESI-MSm / z: 327 [M+Na] + ;HRMS: m / z[M+Na] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com