Detection method and kit of HLA (Human Leukocyte Antigen)-B*58:01 allele
An HLA-B, allele technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve the problems of cumbersome operation, high cost, and long time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] HLA-B*58:01 allele detection kit (SSP-PCR fluorescent probe method) and its use.
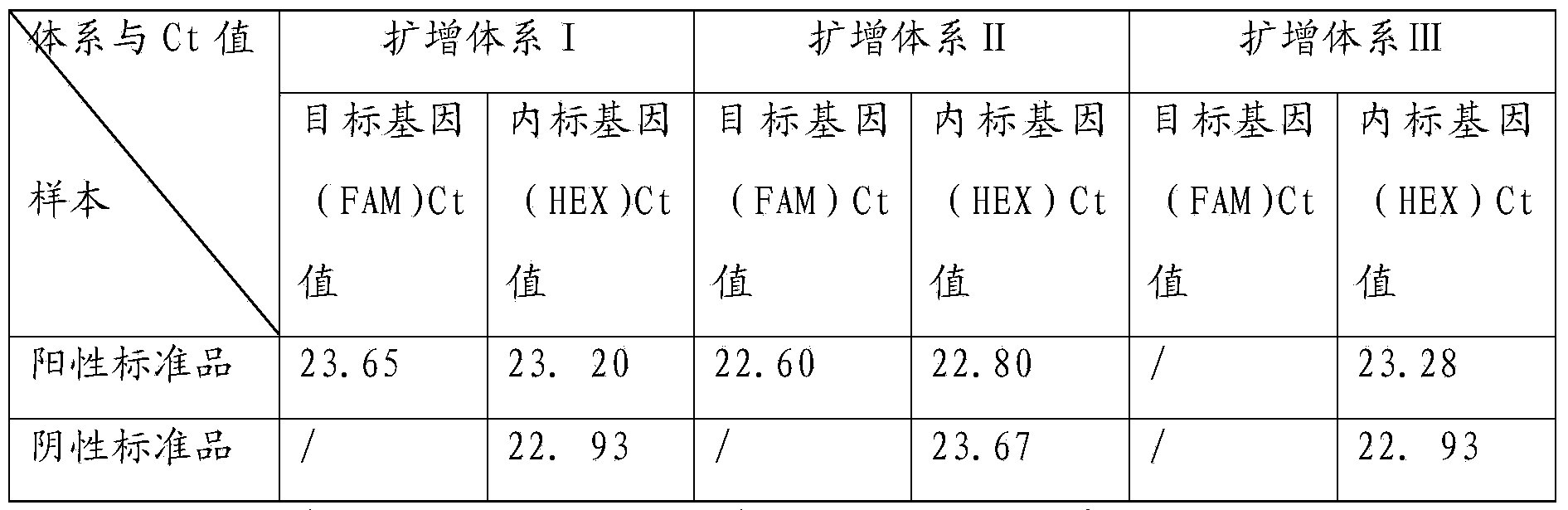
[0058] (1) Prepare a kit including the following components: 1 tube of reaction solution 1 (650μl / tube), 1 tube of reaction solution 2 (650μl / tube), 1 tube of reaction solution 3 (650μl / tube), EZ Taq enzyme mixture (150μl / tube) 1 tube, standard solution 1 (30μl / tube), standard solution 2 (30μl / tube).
[0059] (2) Sample collection, transportation and storage: use a disposable vacuum blood collection device, perform aseptic operation, take blood samples from individuals to be tested, and place the blood samples in a low-temperature refrigerator (-70°C or -20°C) after labeling Store in the freezer. For centralized inspection, specimens must be transported in an environment below 0°C and delivered to the laboratory within 24 hours.
[0060] (3) Inspection steps and result analysis:
[0061] Take 300 μl of the blood sample to be tested, extract the DNA in the blood sample (deleted) and standard 1 a...
Embodiment 2
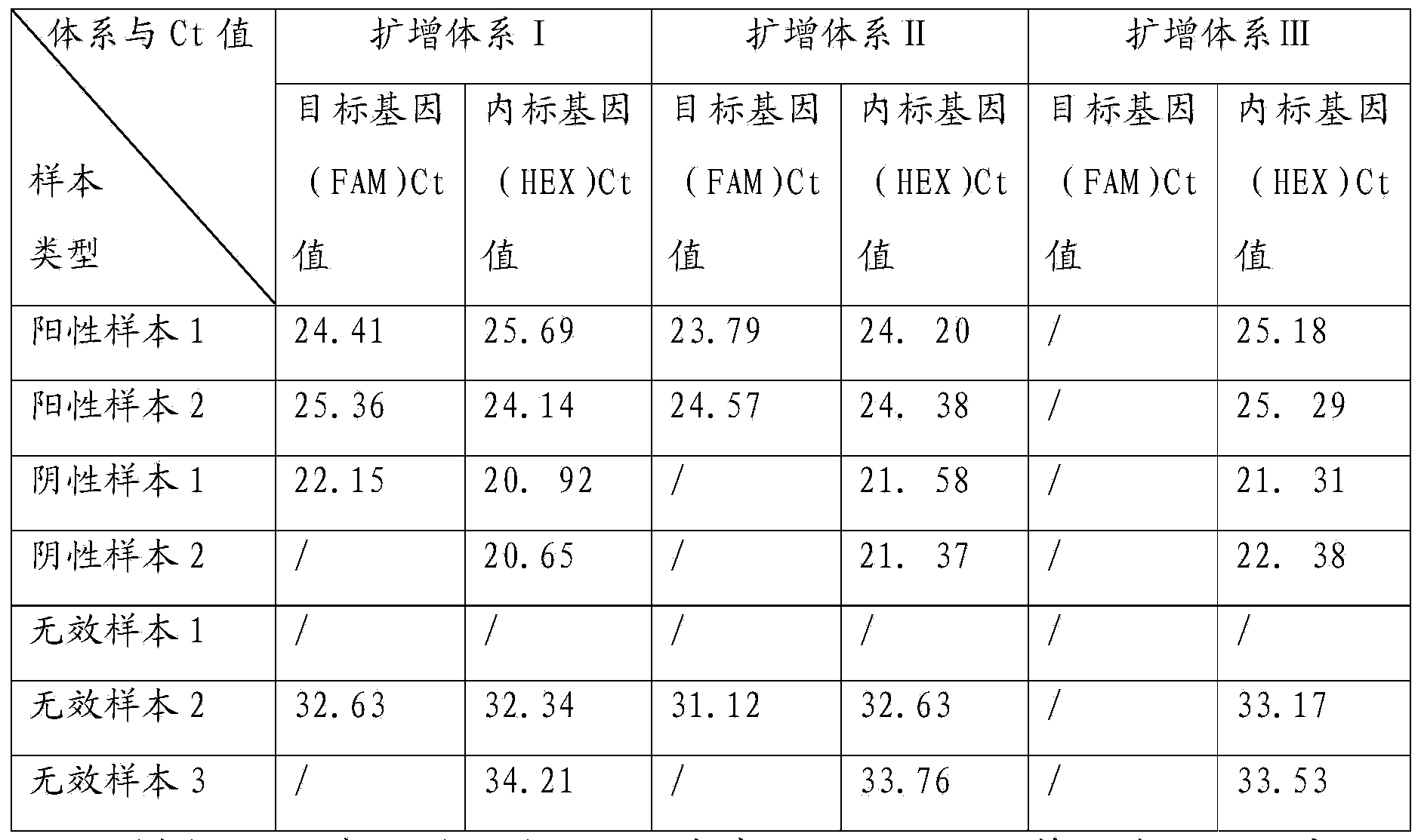
[0071] Example 2: Results and analysis of 30 clinical blood samples detected by the kit of the present invention
[0072] Using the single-blind experiment method, 30 cases were selected from 500 blood samples from Chongqing Blood Center to test with the kit of the present invention, using TBG’s HLAssure ABCDRDQ SBT sequencing kit, using PCR-SBT gold standard method kit Review the results of the experiment. The test data showed that the PCR amplifications of the 30 clinical blood samples tested by this kit were all effective amplifications. Except for No. 1 sample amplification system I and No. 6 sample amplification system III, the target sequences of control genes in other samples were all amplified; except for the amplification system I (Ct34.13) of sample No. 10 and sample No. 17 Except for amplification system I (Ct34.93) and III (Ct34.54), the CT values of the target sequences of the control genes in other samples were all less than 30. Among them, 8 samples had HLA-B*5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com