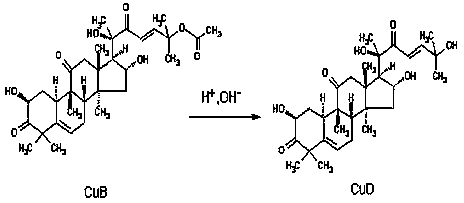
Method for preparing cucurbitacine D by hydrolyzing cucurbitacine B
A technology for cucurbitacin and hydrolyzate, applied in the field of medicine, can solve the problems of single source of cucurbitacin D and low purity, and achieve the effects of easy control of reaction, simple and easy method, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of Cucurbitacin D by Hydrolysis of Cucurbitacin B
[0028] Reaction conditions:
[0029] Cucurbitacin B The organic phase water box pH of aqueous phase organic phase water phase volume ratio temperature reflex Reaction time 100mg 95% ethanol NaOH solution 12 50:50 60℃ 120 minutes
[0030] Weigh 200 mg of cucurbitacin B, dissolve it completely with 20 ml of 95% ethanol, add 20 ml of NaOH solution with a pH value of 12, react at 60°C for 120 min, then stop the reaction in an ice bath, and add 40 ml of The hydrolyzate was extracted with ethyl acetate, and the ethyl acetate was removed by rotary evaporation at 40°C to obtain the desired hydrolyzate.
Embodiment 2
[0031] Example 2: Preparation of Cucurbitacin D by Hydrolysis of Cucurbitacin B
[0032] Reaction conditions:
[0033] Cucurbitacin B The organic phase water box pH of aqueous phase organic phase water phase volume ratio temperature reflex Reaction time 100mg 95% ethanol NaOH solution 10 50:50 60℃ 120 minutes
[0034] Weigh 200 mg of cucurbitacin B, dissolve it completely with 20 ml of 95% ethanol, add 20 ml of NaOH solution with a pH value of 10, react at 60°C for 120 min, then stop the reaction in an ice bath, and add 40 ml of The hydrolyzate was extracted with ethyl acetate, and the ethyl acetate was removed by rotary evaporation at 40°C to obtain the desired hydrolyzate.
Embodiment 3
[0035] Example 3: Preparation of Cucurbitacin D by Hydrolysis of Cucurbitacin B
[0036] Reaction conditions:
[0037] Cucurbitacin B The organic phase water box pH of aqueous phase organic phase water phase volume ratio temperature reflex Reaction time 100mg 95% ethanol NaOH solution 14 50:50 60℃ 120 minutes
[0038] Weigh 200 mg of cucurbitacin B, dissolve it completely with 20 ml of 95% ethanol, add 20 ml of NaOH solution with a pH value of 14, react at 60°C for 120 min, then stop the reaction in ice bath, add 40 ml of The hydrolyzate was extracted with ethyl acetate, and the ethyl acetate was removed by rotary evaporation at 40°C to obtain the desired hydrolyzate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com