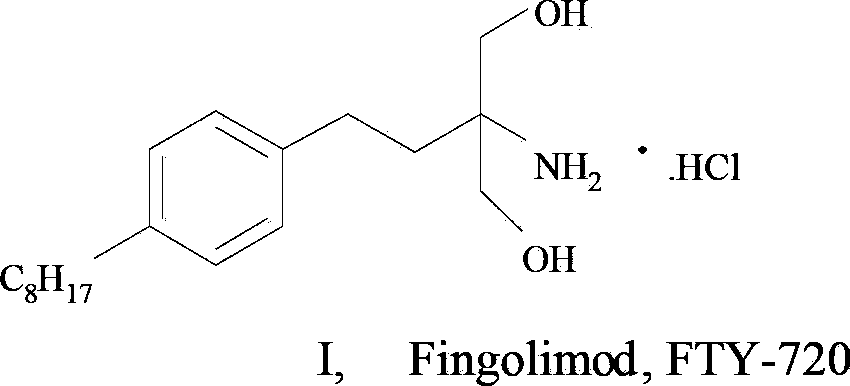
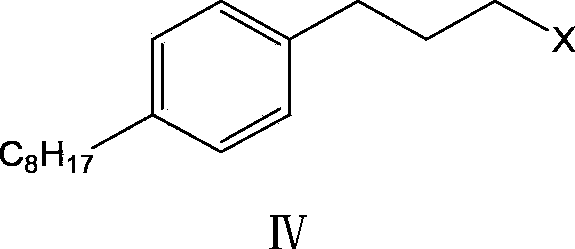
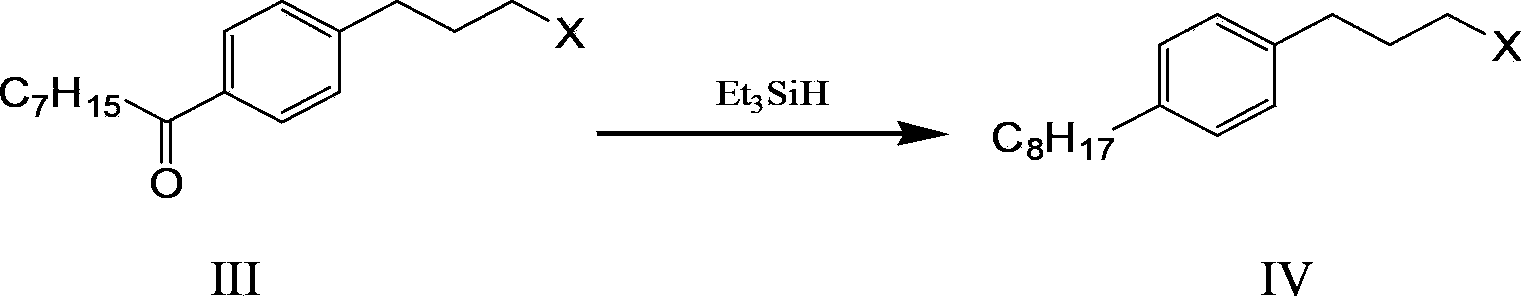
Method and intermediate for synthesizing Fingolimod hydrochloride
A Chinese-style fingolimod hydrochloride technology, applied in the field of drug synthesis, can solve the problems of product purity not clearly stated, too long reaction steps, long reaction routes, etc., and achieve easy industrial scale-up production, short reaction steps, and simplified operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment pair octanoyl bromide propyl benzene (compound III)
[0068] Add aluminum trichloride (160g, 1.2mol, 2eq) into a 500ml three-necked flask, keep the temperature of the reaction solution not higher than -15°C, and add the pre-mixed 3-bromophenylpropane (121g, 0.6mol, 1eq), and n-octanoyl chloride (116g, 0.72mol, 1.2eq). After the dropwise addition, stir at -5°C until the reaction is complete [reaction takes about 4 hours, HPLC detection: raw material 3-bromophenylpropane ≤ 0.5%]. When the mixed solution is kept at 0°C-10°C, slowly pour the reaction solution into 1200ml of 5% hydrochloric acid water, extract with ethyl acetate (200ml×3 times), combine the organic layers, wash the organic layer with saturated brine, anhydrous sodium sulfate (50g ), dried, filtered, and the filtrate was concentrated by rotary evaporation at no higher than 40°C to obtain p-octanoyl bromide propylbenzene (190 g, HPLC purity 96.93%, yield 97%) as a yellow oil. h ...
Embodiment 2
[0069] The preparation of embodiment two p-octanoyl bromide propyl benzene (compound III)
[0070] Add aluminum trichloride (160g, 1.2mol, 2eq) into a 500ml three-neck flask, keep the temperature of the reaction solution not higher than -15°C and add 3-bromophenylpropane (121g, 0.6mol, 1eq) dropwise with stirring, then Keep the reaction temperature below -20°C and add n-octanoyl chloride (116g, 0.72mol, 1.2eq) dropwise. After the dropwise addition, stir at below -5°C until the reaction is complete [reaction takes about 4 hours, HPLC detection: raw material 3-bromo Phenylpropane≤0.5%]. When the mixed solution is kept at 0°C-10°C, slowly pour the reaction solution into 1200ml of 5% hydrochloric acid water, extract with ethyl acetate (200ml×3 times), combine the organic layers, wash the organic layer with saturated brine, anhydrous sodium sulfate (50g ), dried, filtered, and the filtrate was concentrated by rotary evaporation at no higher than 40°C to obtain p-octanoyl bromide p...
Embodiment 3
[0071] The preparation of embodiment three p-octanoyl iodopropylbenzene (compound III)
[0072] Add aluminum trichloride (5.3g, 0.04mol, 2eq) into a 50ml three-necked flask, keep the temperature of the reaction solution not higher than -15°C and add 3-iodophenylpropane (5g, 0.02mol , 1 eq) and n-octanoyl chloride (3.9 g, 0.024 mol, 1.2 eq). After the dropwise addition, stir at -5°C until the reaction is complete [the reaction takes about 2-4 hours, HPLC detection: raw material 3-iodophenylpropane ≤ 0.5%,]. Keep the mixed solution at 0°C-10°C, slowly pour the reaction solution into 50ml of 5% hydrochloric acid water, extract with ethyl acetate (20ml×3 times), combine the organic layer, wash the organic layer with saturated brine, anhydrous sodium sulfate (3g) After drying and filtering, the filtrate was concentrated by rotary evaporation at no higher than 40°C to obtain p-octanoyl iodopropylbenzene (6.8 g, HPLC purity 96.26%, yield 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com