Cryopreservation resuscitation solution for sweat gland-like cells and cryopreservation and resuscitation method for maintaining cell activity
A technology of sweat gland-like cells and resuscitation fluid, applied in the field of human sweat gland regeneration research, can solve the problems of low survival efficiency and unfavorable resuscitation applications, and achieve the effects of clear components, high survival rate, and easy use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Cell cryopreservation
[0035] Preparation of cryopreservation solution: The components of the cryopreservation solution used in this example are: the base fluid serum substitute contains:
[0036] Human epidermal growth factor at a concentration of 70 ng / mL, recombinant human keratinocyte growth factor-II at a concentration of 40 ng / mL, triiodothyronine at a concentration of 25 ng / mL, hydrocortisone hemisuccinate at a concentration of 0.7 μg / mL, insulin-transferrin-sodium selenite, the concentration is 0.05mL / mL, bovine serum albumin, the concentration is 0.4g / mL, and the prepared cryopreservation solution is pre-cooled in a refrigerator at 4°C for 30 minutes .
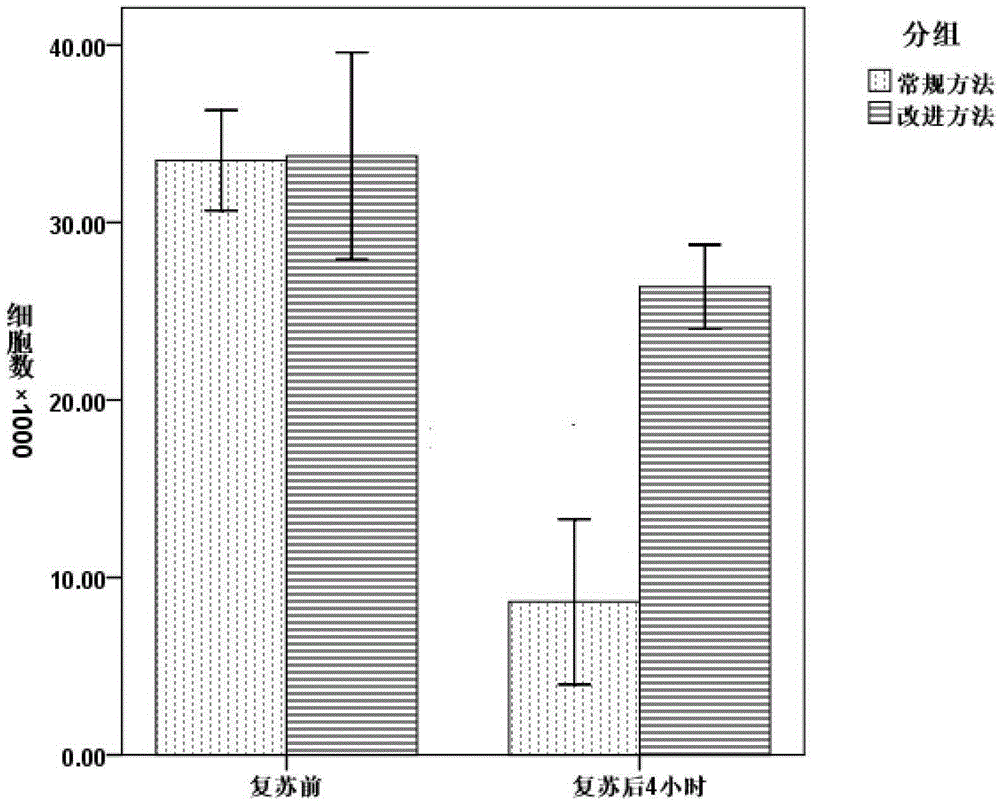
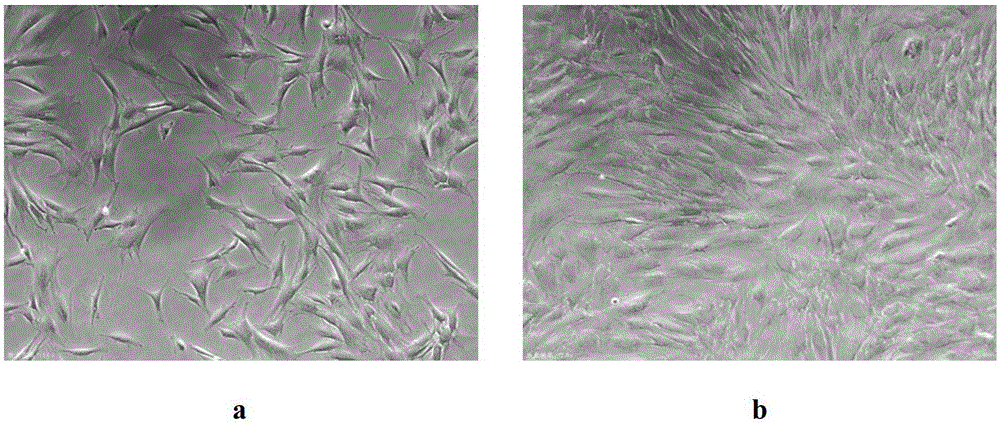
[0037]Take the induced sweat gland-like cells, digest them, collect them and centrifuge them, then add the pre-cooled cryopreservation solution so that the cell concentration is 0.5-2×10 6 / mL, transfer to cryopreservation tubes at 1.5mL / branch, and pre-cool at 4°C for 30 minutes. Put it into the temper...
Embodiment 2
[0047] (1) Cell cryopreservation
[0048] The composition of the cryopreservation solution used in this example is: the base solution Knock-out serum substitute contains:
[0049] Human epidermal growth factor at a concentration of 80 ng / mL, recombinant human keratinocyte growth factor-II at a concentration of 50 ng / mL, triiodothyronine at a concentration of 30 ng / mL, hydrocortisone hemisuccinate at a concentration of 0.8 μg / mL, insulin-transferrin-sodium selenite, the concentration is 0.1mL / mL, bovine serum albumin, the concentration is 0.6g / mL.
[0050] The cryopreservation operation is the same as in Example 1.
[0051] (2) Cell recovery
[0052] The components of the resuscitation fluid used in this example are: the base fluid Knock-out serum substitute contains: human epidermal growth factor at a concentration of 80 ng / mL, recombinant human keratinocyte growth factor-II at a concentration of 50 ng / mL, triiodine Thyronine at a concentration of 30 ng / mL, hydrocortisone h...
Embodiment 3
[0057] (1) Cell cryopreservation
[0058] The composition of the cryopreservation solution used in this example is: the base solution Knock-out serum substitute contains:
[0059] Human epidermal growth factor at a concentration of 75 ng / mL, recombinant human keratinocyte growth factor-II at a concentration of 45 ng / mL, triiodothyronine at a concentration of 27 ng / mL, hydrocortisone hemisuccinate at a concentration of 0.75 μg / mL, insulin-transferrin-sodium selenite, the concentration is 0.07mL / mL, bovine serum albumin, the concentration is 0.4g / mL.
[0060] The cryopreservation operation is the same as in Example 1.
[0061] (2) Cell recovery
[0062] The composition of the resuscitation fluid used in this embodiment is: the base fluid Knock-out serum substitute contains:
[0063] Human epidermal growth factor at a concentration of 75 ng / mL, recombinant human keratinocyte growth factor-II at a concentration of 45 ng / mL, triiodothyronine at a concentration of 27 ng / mL, hydro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com