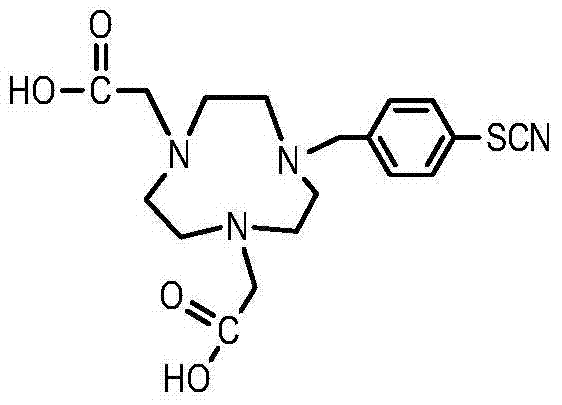
Method for synthesizing bifunctional chelating agent p-SCN-NODA (1,4,7-triazacyclooctane-1,4-diacetic acid-7-p-isothiocyanobenzyl)
The technology of a bifunctional chelating agent and a synthesis method, which is applied in the field of medicinal chemistry, can solve the problems of high preparation cost and poor economy, and achieve the effects of simple operation, guaranteed quality and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthetic method of bifunctional chelating agent p-SCN-NODA described in the present embodiment comprises the steps:
[0040] (1) Add 1,4,7-triazacycloctane I (3.0g, 23.23mmol) and 30ml of chloroform into a round bottom flask, then dissolve tert-butyl bromoacetate (9.97g, 51.11mmol ) of the chloroform solution 60ml was slowly added dropwise to the round bottom flask (the dropping time was 1 hour); the molar ratio of the tert-butyl bromoacetate and the 1,4,7-triazacycloctane I It is 2.2:1. After stirring overnight at room temperature, the solvent was evaporated to dryness, the remaining residue was dissolved in 15ml of water, the pH value was adjusted to 3.0 with 0.1M HCl, and the by-products were removed by extraction twice with 30ml of anhydrous ether; Add 0.1M NaOH in the remaining aqueous phase to carry out alkalization, the aqueous phase after alkalization is white milky, then extract 2 times with 50ml dichloromethane, collect organic phase, dichloromethane ext...
Embodiment 2
[0046] The synthetic method of bifunctional chelating agent p-SCN-NODA described in the present embodiment comprises the steps:
[0047] (1) Add 1,4,7-triazacycloctane I (3.14g, 24.33mmol) and 32ml of chloroform into a round bottom flask, then dissolve tert-butyl bromoacetate (9.97g, 51.11mmol ) of chloroform solution 60ml was slowly added dropwise to the round bottom flask (the dropping time was 1 hour), the molar ratio of the tert-butyl bromoacetate and the 1,4,7-triazacycloctane I is 2.1:1. After stirring overnight at room temperature, the solvent was evaporated to dryness, the remaining residue was dissolved in 15ml of water, the pH value was adjusted to 3.5 with 1M HCl, and the by-products were removed by extraction twice with 30ml of anhydrous ether; Add 1M NaOH in the remaining aqueous phase and carry out alkalization, the aqueous phase after alkalization is white milky, then extract 2 times with 50ml methylene chloride, collect organic phase, dichloromethane extract i...
Embodiment 3
[0054] The synthetic method of bifunctional chelating agent p-SCN-NODA described in the present embodiment comprises the steps:
[0055] (1) Add 1,4,7-triazacycloctane I (2.87g, 22.22mmol) and 30ml of chloroform into a round bottom flask, then dissolve tert-butyl bromoacetate (9.97g, 51.11mmol ) of chloroform solution 60ml was slowly added dropwise to the round bottom flask (the dropping time was 1 hour), the molar ratio of the tert-butyl bromoacetate and the 1,4,7-triazacycloctane I It is 2.3:1. After stirring overnight at room temperature, the solvent was evaporated to dryness, the remaining residue was dissolved in 15 ml of water, the pH value was adjusted to 3.2 with 0.5 M HCl, and the by-products were removed by extraction twice with 30 ml of anhydrous ether; Add 0.5M NaOH to the remaining aqueous phase after extraction for alkalization, the aqueous phase after alkalization is white and milky, then extract twice with 50ml dichloromethane, collect the organic phase, conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com