Solid forms of transthyretin dissociation inhibitors
A form, crystal technology, applied in the field of solid forms of transthyretin dissociation inhibitors, which can solve the problem of incomparability of accurate dosage forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
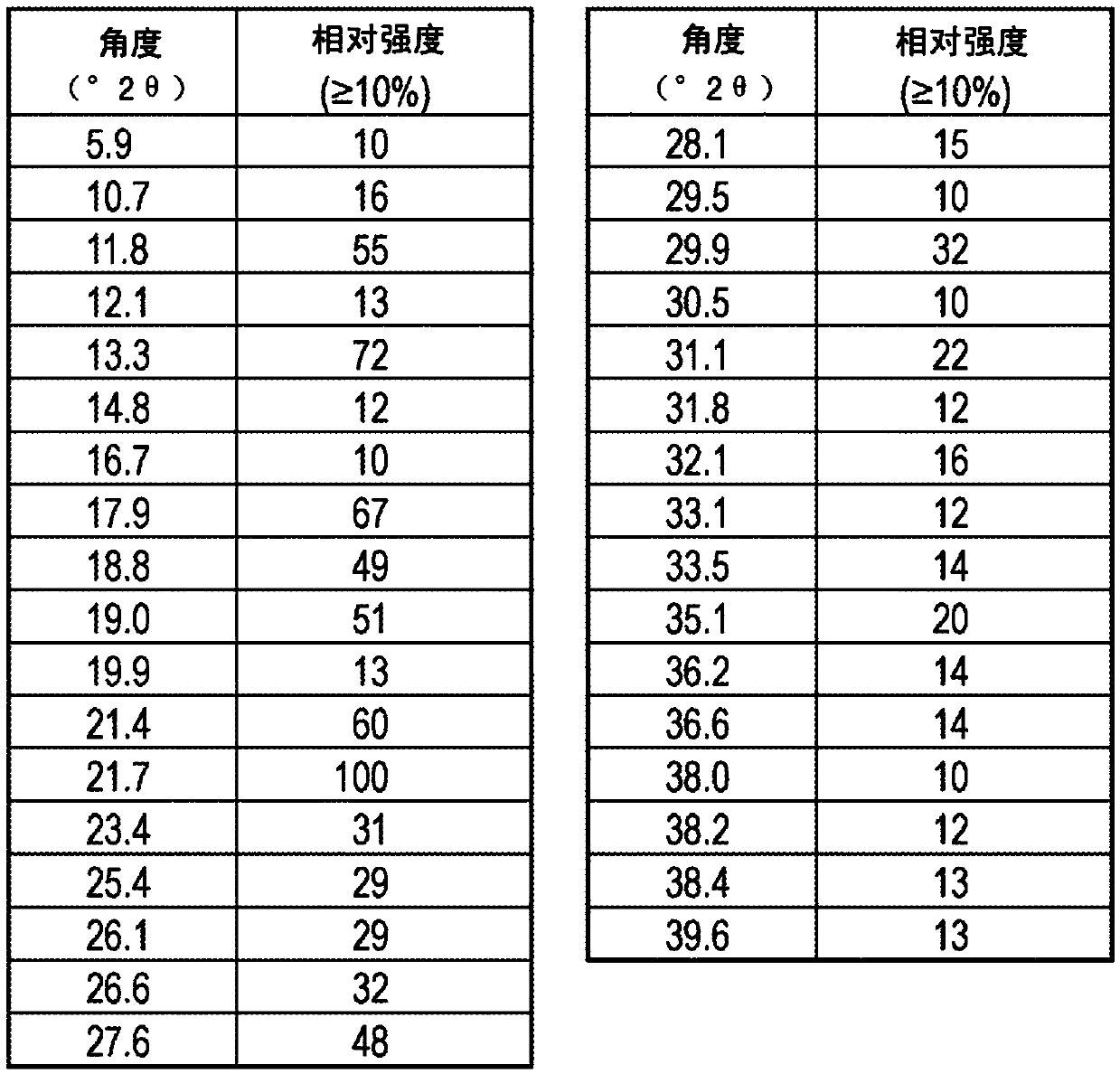
[0137] Embodiment 1--the preparation of crystal compound 1
[0138] 6-Carboxy-2-(3,5-dichlorophenyl)-benzoxazole free acid (2.5 g, 8.1 mmol) and 2-propanol (49 mL) were added to a 100 mL jacketed flask with a magnetic stirrer 2 neck round bottom flask. The resulting slurry was warmed to 70°C with stirring. Water (8.8 mL) was then added. In a separate 15 mL round bottom flask, a solution of N-methyl-D-glucamine (1.58 g, 8.1 mmol) in 5 mL of water was prepared and dissolved with stirring. The aqueous N-methyl-D-glucamine solution was then transferred to the reaction flask over 2 minutes. At the end of this addition, most (but not all) of the solids had dissolved. After stirring for 5 minutes and warming to 79°C a light yellow solution was obtained. Pass the solution through Celite TM The layer was filtered, cooled to 60°C, then to 10°C over 2 hours. The resulting solid was collected by filtration and washed with 10 mL of 2-propanol. 3.35 g of product were obtained (82% y...
Embodiment 2
[0139] Embodiment 2--The preparation of compound 1 liquid crystal
[0140] Crystalline Compound 1 (505 mg) was dissolved in 60 mL of water at room temperature. Transfer the solution to a lyophilization vessel and freeze with rotation in an acetone / dry ice bath. The vessel was transferred to a benchtop lyophilizer and allowed to dry under vacuum for approximately 19 hours to yield a white solid.
Embodiment 3
[0141] Embodiment 3--preparation of amorphous compound 1
[0142] Crystalline Compound 1 (approximately 500 mg) was transferred to an aluminum pan and placed on a hot plate at 200°C. Melting occurred within 1 min, at which point the pan was removed from the heating plate and placed immediately in liquid nitrogen. A glassy solid was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com