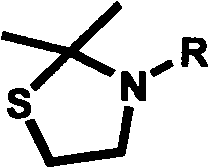
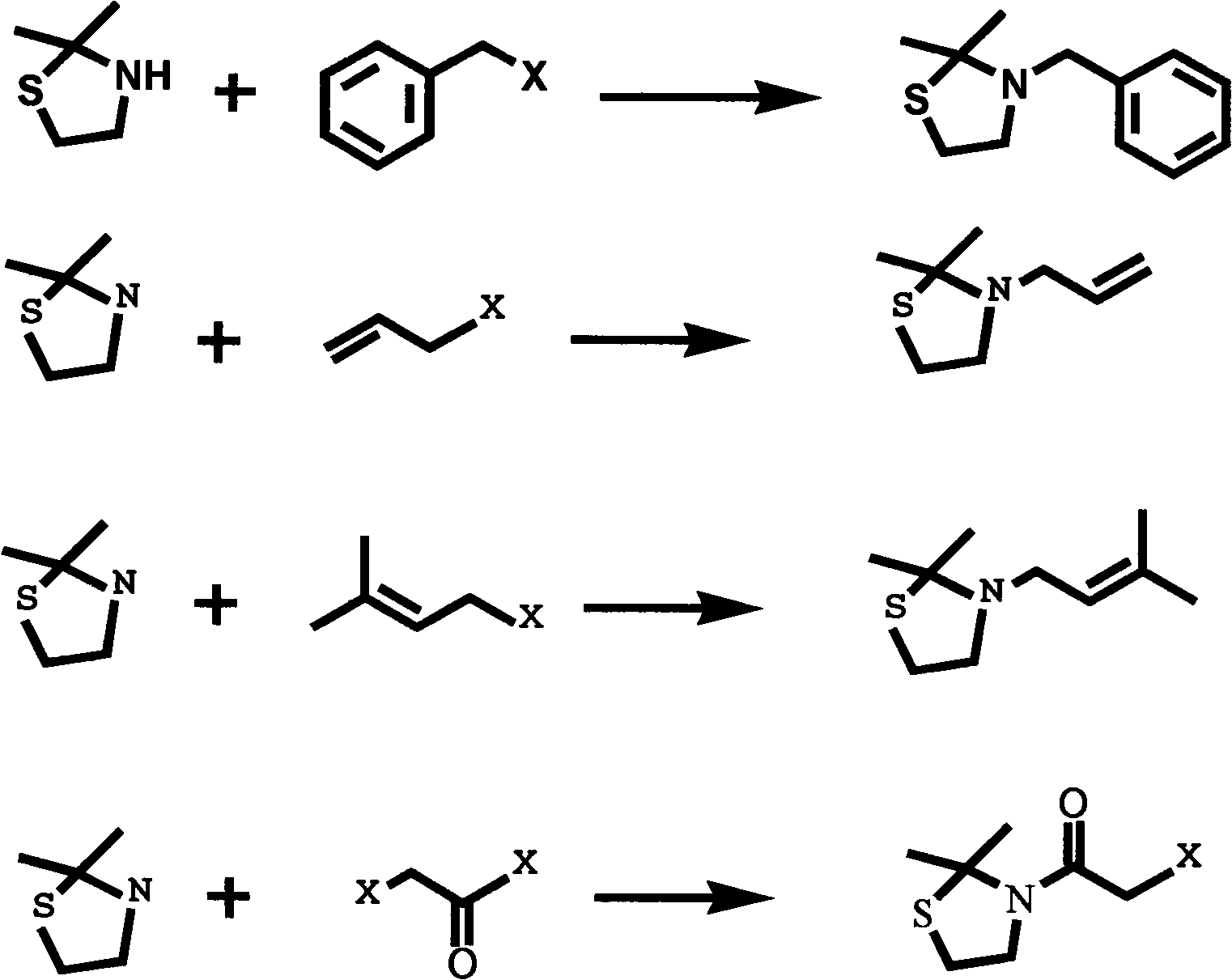
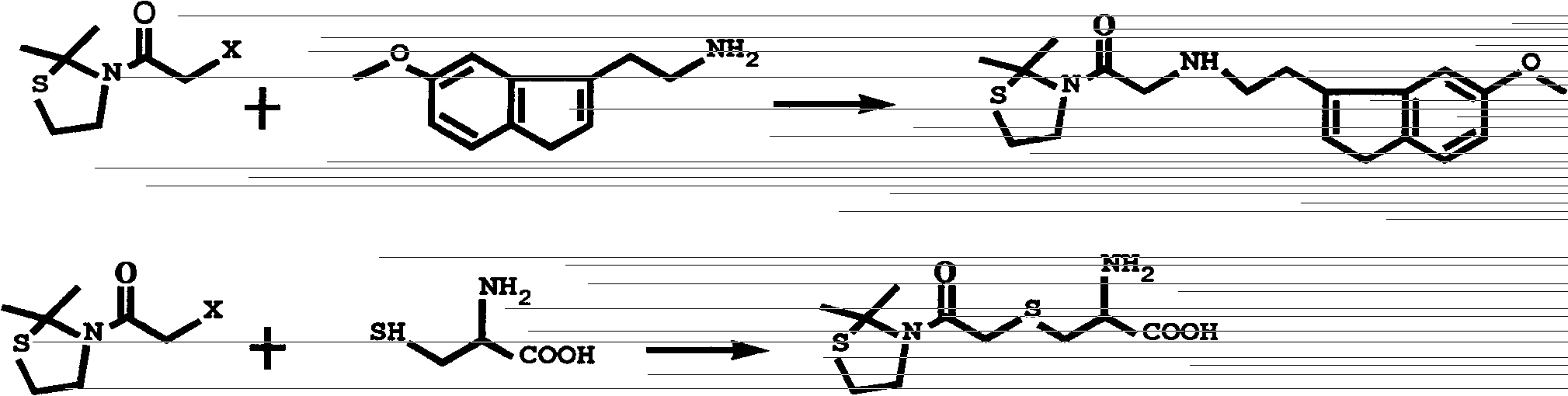
Substituted 2,2-dimethylthiazolidin compound, as well as preparation method and use thereof
A technology of dimethyl thiazolidine and compound, which is applied in the directions of medical preparations containing active ingredients, organic chemistry, and drug combinations, etc., can solve the problems of poor drugability and inconvenient administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: 2-chloro-1-(2,2-dimethyl-3-thiazolidinyl)ethanone
[0054] Weigh 10mmol of 2,2-dimethylthiazolidine and dissolve it in 10ml of acetone, add an appropriate amount of anhydrous sodium acetate under stirring at room temperature, add dropwise 12mmol of chloroacetyl chloride diluted with acetone under ice bath conditions, after the dropwise addition React for 2h, then gradually increase the temperature to 40°C and continue to react for 2h. After the reaction is finished, use a rotary evaporator to spin off the solvent to obtain a pink powder, and add an appropriate amount of CHCl 3 Dissolve, wash with water, dilute acid, dilute alkali and salt to get light yellow CHCl 3 solution, spin off CHCl 3 solvent to give crude product. The crude product was recrystallized with petroleum ether (60-90° C.), and the product was white needle-like crystals with a yield of 89.2%. mp66.2~67.8℃. MS: Calculated value [C7 h 12 NOSC1] + : 193.5, test value [C 7 h 12 NOSC1] +...
Embodiment 2
[0055] Example 2: 2-amino-3-[2-(2,2-dimethyl-3-thiazolidinyl)-2-oxoethylmercapto]propionic acid
[0056] Add 12mmol of L-cysteine to a 100ml reaction bottle, and add 12mmol of L-cysteine at 3mol / LNH 4 After 15ml of OH is dissolved, control the temperature below 3°C in an ice bath, and add dropwise 10mmol of 2-chloro-1-(2,2-dimethyl-3-thiazolidinyl) dissolved in 10mL of absolute ethanol under vigorous stirring ) ethyl ketone, maintaining the reaction temperature to continue the reaction for 4h. Then the solvent was evaporated to dryness under reduced pressure (2 O / C 2 h 5 OH recrystallized to obtain white fine needle crystals with a yield of 74.2%. mp 178.3-179.7°C. MS: Calculated value [C 10 h 18 N 2 o 3 S 2 +H] + : 279.0832, test value [C 10 h 18 N 2 o 3 S 2 +H] + : 279.0830. 1 H-NMR (300MHz, D 2 O) δppm: 3.83 (m, 2H), 3.74 (m, 1H), 3.42 (s, 2H), 2.96 (m, 4H), 1.62 (s, 6H). IR: 2977, 2909 (v as , v s CH 3 ), 1632 (v as C=O), 3273, 964 (v, γOH), ...
Embodiment 3
[0057] Example 3: 1-(2,2-Dimethyl-3-thiazolidinyl)-2-[2-(5-methoxy-3-indolyl)ethylamino]ethanone
[0058] Weigh 5.25mmol 5-methoxytryptamine and dissolve it in 20ml THF, add an appropriate amount of catalyst KI and 6mmolK 2 CO 3 , stirred and dissolved at 40°C, then 5mmol 2-chloro-1-(2,2-dimethyl-3-thiazolidinyl)ethanone dissolved in 10ml THF was added dropwise, and reacted at reflux at 70°C for 6h. After the reaction is completed, the solvent is spin-off with a rotary evaporator to obtain a gray powder, and an appropriate amount of CHCl is added 3 Dissolve, wash with water, spin off CHCl 3 Add appropriate amount of ethyl acetate and stir at 40°C, filter to obtain a gray solid, and wash the filter cake with water and ethyl acetate, respectively. The obtained crude product was recrystallized in acetone to obtain earth gray flaky crystals with a yield of 58.4%. mp 166.8-168.5°C. MS: Calculated value [C 18 h 25 N 3 o 2 S] + :347, test value [C 18 h 25 N 3 o 2 S] + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com